Research Roundup: ESwab® Can Do All That?!
February 11, 2022

Welcome to Research Roundup! Each month COPAN will feature a comprehensive review and commentary on recent scientific publications covering a particular topic of interest. COPAN’s Research Roundup is authored by Dr. Susan Sharp, COPAN’s Scientific Director!
In this month’s edition, Dr. Sharp summarizes publications on ESwab® and outlines the wide range of applications documented since the sample collection and transport system’s debut in 2006.
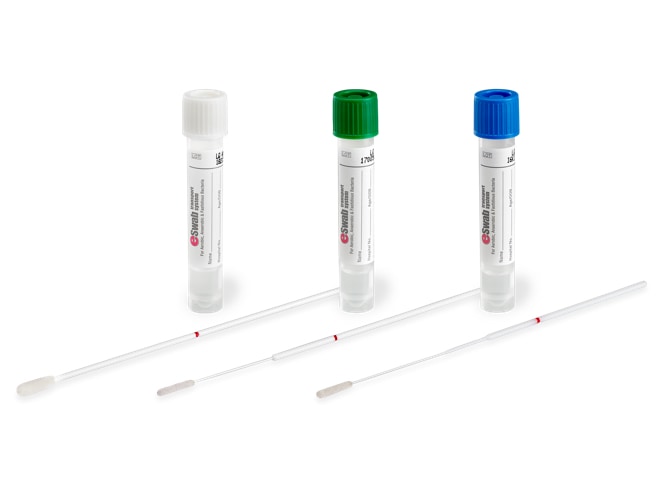
What is ESwab®?
ESwab® is a patented liquid-based collection and transport system for Microbiology samples that combines a COPAN-invented flocked swab with 1mL of Liquid Amies media in a plastic, screw cap tube. The innovative system elutes over 90% of the patient specimens into the liquid medium. The results are evidence-based and show improved pathogen recovery and expanded testing capabilities leading to improved patient care.
ESwab® gained FDA clearance in June 2006 and began to revolutionize sample collection and diagnostic testing in clinical bacteriology. ESwab™ has led an entirely new approach to Liquid-Based Microbiology with its versatility and multipurpose capability. ESwab™ quickly became the mainstay and go-to device for sample collection. This became even more apparent during the COVID-19 pandemic. This Research Roundup summarizes recent publications on ESwab™ and outlines the beneficial applications documented since the sample collection and transport system’s debut in 2006.
Scientific Review of Publications
ESwab® for COVID-19 Diagnosis

The use of ESwab® was accepted early in the pandemic by the Food and Drug Administration (FDA) as an alternative collection device for COVID-19 diagnosis. This recommendation remains in effect according to the FDA’s last update of 11/15/21 which states: “The best available evidence at this time indicates that the liquid Amies will stabilize the SARS-CoV-2 RNA”. Many studies have been published showing the effectiveness of ESwab® for use with COVID-19 diagnostic tests.
Comparing ESwab® vs UTM®
A study by Deiana et al., used 3 mL UTM®: Universal Transport Medium™ and ESwab® to evaluate the performance of the 2019-nCoV CDC ddPCR quantitative Triplex Probe Assay from BioRad. A total of 50 nasopharyngeal swabs (25 placed into UTM® and 25 placed into ESwab®) from SARS-CoV-2 patients were used. Their direct quantitation results showed that the ESwab® samples were more accurate with a sensitivity of 93.33% and specificity of 100.00% for both N1 and N2 COVID-19 targets and that there was a lower rate of discordant results for N1 and N2 with the ESwab®.
Study Conclusion
Results from the ESwab® were equal to or exceeded those of UTM® in terms of sensitivity and specificity.
Comparing Nasopharyngeal (NP) Swabs vs. Oropharyngeal/Nasal (OP/N) Swab Collections
The study by Desmet et al., evaluated the use of nasopharyngeal (NP) swabs vs. oropharyngeal/nasal (OP/N) swab collections for the diagnosis of COVID-19. NP samples were added to either UTM® or ESwab® and OP/N samples were added to ESwab®. The study is the first to indicate that the molecular detection of SARS-CoV-2 with combined OP/N swabs was non-inferior to NP sampling. In addition, the study found a high correlation between the viral loads of the paired OP/N and NP samples, with the sensitivities of NP and OP/N specimens in hospitalized patients of 89.3% and 92.7%.
Study Conclusion
The study is the first to indicate that the molecular detection of SARS-CoV-2 with combined OP/N swabs was non-inferior to NP sampling. In addition, the study found a high correlation between the viral loads of the paired OP/N and NP samples.
Comparing Nasopharyngeal (NP) COVID-19 samples in UTM® to Mid-Turbinate (MT) samples
Vermeiren et al., compared NP samples in UTM® to mid-turbinate samples using ESwab® on 94 patients on two PCR platforms finding no discrepant results. Comparing the two types of swabs, the positive percent agreement in both assays was between 97.1-100% and the negative percent agreement was 100% for both assays. For positive swabs, the average cycle threshold (CT) values for each of the SARS-CoV-2 targets did not show a statistically significant difference between UTM® swabs and ESwab®.
Study Conclusion
For positive swabs, the average cycle threshold (CT) values for each of the SARS-CoV-2 targets did not show a statistically significant difference between UTM® swabs and ESwab®. The negative percent agreement was 100% for both assays.
Comparing Mid-Turbinate (MT) Samples vs Oropharyngeal (OP) Covid-19 Samples
Finally, the study by Palmas et al., using ESwab® for all collections demonstrated significantly higher positivity rates of mid-turbinate swab testing over OP swab testing in detecting SARS-CoV-2 in COVID-19 positive children.
Study Conclusion
ESwab® showed significantly higher positivity rates of MT swab testing over OP swab testing
ESwab® Suitablility for Aerobic, Anaerobic and Fastidious Bacteria

ESwab® has been used for many years prior to the COVID-19 pandemic. ESwab® has been tested and validated in full compliance with CLSI M40-A2: Quality Control of Microbiological Transport System Standard. The FDA cleared collection and transport system is suitable for aerobic, anaerobic and fastidious bacteria maintaining viability for up to 48 hours at room or refrigerator temperature. Many studies have been published on the uses of the versatile system.
ESwab® for Rectal and Throat ESBL-Enterobacterales Specimens
Andremont et al., evaluated whether a semiquantitative assessment of rectal and throat ESBL-Enterobacterales carriage could predict ESBL-ventilator-associated pneumonia (VAP) in medical ICU patients. ESwab® was used for collection of both the rectal and the throat specimens. This methodology allowed them to determine that in carriers of ESBLs other than E. coli, ESBL throat carriage or a high-density ESBL rectal carriage are risk factors for the development of ESBL-associated VAP.
Study Conclusion
Using ESwab® allowed researchers to determine that in carriers of ESBLs other than E. coli, ESBL throat carriage or a high-density ESBL rectal carriage are risk factors for the development of ESBL-associated VAP.
ESwab® for Skin Microbiome Analyses
Bjerre et al., undertook a study to establish a methodology for skin microbiome analyses focusing on sampling technique and DNA extraction. The authors compared skin specimens collected with ESwab® to skin scrapings taken from healthy adults in 12 commercial DNA extraction kits.
Study Conclusion
The study showed that there were no differences in concentrations of isolated DNA or success of library preparation between ESwab® and skin scrapes, and state that ESwab® may be preferable as they provide more consistent results and are a less invasive collection technique.
View the video below showing how to properly collect a wound sample using ESwab®
ESwab® for Viral Surveillance of Surfaces
Casini et al., used ESwab® to sample endoscopes and gastroscopes used on COVID-19 patients in order to access that they were free of the virus after reprocessing steps and prior to reuse.
Study Conclusion
The improvement in endoscope reprocessing implemented during the COVID-19 emergency was effective in ensuring the absence of SARS-CoV-2, thus reducing the risk of infections after an endoscopy on COVID-19 patients.

ESwab® with COPAN WASP® for Detection of Common Female Reproductive Pathogens
Gao et al., explored the potential clinical application of ESwab® used with COPAN WASP® automated processing system in the detection of pathogens in female reproductive tract specimens and its feasibility in optimizing diagnostic procedures.
Study Conclusion
Researchers concluded that ESwab® used on the WASP® facilitated the optimization of diagnostic procedures for the detection of common pathogens in the female reproductive system, thereby reducing associated costs.

WASP®DT: Walk-Away Specimen Processor
WASP®DT is a modular, open platform specimen processing solution that fully automates all facets of specimen set-up in Microbiology, including planting and streaking, Gram slide preparation, broth inoculation and ID disk application.
ESwab® for Maintaining Aerobic Bacteria from Rectal Swab Sampling During -80C Freezing
Saliba et al., evaluated the performance of ESwab® for maintaining the survival of aerobic bacteria from rectal swab sampling during -80oC freezing. The study observed complete stability of the quantification of E. coli isolates during a three-month freezing period and confirmed that ESwab® can be used not only for molecular bacterial tests, but also for the maintenance of bacterial viability in clinical specimens.
Study Conclusion
The study observed the complete stability of the quantification of E. coli isolates during a three-month freezing period and confirmed that ESwab® can be used both for molecular bacterial tests and the maintenance of bacterial viability.
ESwab® for Recovery of Aerobic Gram-Negative Bacteria after Long-Term Storage
Sofie et al., evaluated the ESwab® transport system for the quantitative recovery of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa after 1, 2, 3, 5, and 7 days of storage at room and refrigerator temperatures, and at 7 and 30 days of storage at −80 °C and −20 °C using mono- and polymicrobial samples. The study showed that the ESwab® met CLSI standards at room and refrigerator temperatures for all combinations of bacterial strains tested. At −80°C they showed bacterial viability was maintained in monomicrobial samples; however, in polymicrobial samples, P. aeruginosa recovery was compromised; and that storage at −20 °C was unsuitable. The study concluded that specimens collected using ESwab® should be transported to the laboratory as soon as possible and that if transport or processing is delayed, specimens should be stored at refrigerator temperatures.
Study Conclusion
The study showed that the ESwab® met CLSI standards at room and refrigerator temperatures for all combinations of bacterial strains tested and concluded that, if transport or processing is delayed, specimens should be stored at refrigerator temperatures.
ESwab® for Routine MRSA Screening
Von Allmen et al., compared different swab collection systems for routine nasal–throat MRSA screening in patients over three consecutive months. Each month a different type of swab was used: dry swabs (month 1), ESwab® (month 2), and MSwab™ (month 3). MRSA was tested using 3 different PCR assays and chromogenic culture. Among 1,680 subjects, the MRSA detection rate did not differ significantly between dry swabs, ESwab®, and MSwab™ (6.0%, 6.2%, and 5.3%, respectively), and detection rates using chromogenic culture were lower at 2.9%, 3.9%, and 1.9%, respectively.
Study Conclusion
Researchers concluded that efficient and accurate MRSA screening can be achieved using all 3 collection systems, and that specimen collection using ESwab® or MSwab® can facilitate efficient processing on chromogenic culture in full laboratory automation and allows for testing on molecular platforms.
ESwab® as a Multipurpose Swab Sample Collection and Transport System
A Single ESwab® Providing Multiple Uniform Samples
- As exemplified in collection guidance from the Cleveland Clinic, ESwab® a “The ESwab® system generates 1 mL of patient sample, providing a uniform sample for culture and reducing the need to collect multiple swabs.”
- Clin Lab Navigator reminds us, “ESwab® elutes the entire sample into the medium providing up to 10 identical 100µL aliquots of liquid sample suspension so you can run multiple tests from the same specimen”.
- Rapid Microbiology states, “One millilitre of patient sample in every ESwab® tube expands the possible number of first line tests with extra volume in reserve for reflex analysis or sample retention for further investigations”.
A Single ESwab® Used for Multiple Tests
The ability of the ESwab® to allow for additional tests without the need for additional collections has also been reported.
- The Van et al. study showed the versatility of the ESwab® in that it allowed for eight different tests to be performed from one ESwab® collection.
- A study by Fontana et al. concludes that the ESwab® provides a unique collection device for several investigations and also guarantees quality due to the uniformity of the sample and standardization of procedures. patient sample in every ESwab® tube expands the possible number of first line tests with extra volume in reserve for reflex analysis or sample retention for further investigations”.
Further Research on ESwab® Versatility
ESwab® has been shown to be acceptable for the transport and storage of anaerobic, fungal, and mycobacterial organisms, further emphasizing the versatility of this collection device.
- Comparison of the Copan ESwab® System with an Agar Swab Transport System for Maintenance of Fastidious Anaerobic Bacterium Viability
- Evaluation of the Copan ESwab® Transport System for Viability of Pathogenic Fungi by Use of a Modification of Clinical and Laboratory Standards Institute Document M40-A2
- Recovery of Nontuberculous Mycobacteria and Nocardiae Causing Skin/Soft Tissue Infections by Use of the Copan ESwab® Collection and Transport System
The Roundup: Conclusions on the Research on ESwab®

Whether dealing with the COVID-19 pandemic, finding better ways to help in the diagnosis of other infections, collecting the best specimens for routine screening, or providing specimen volume for multiple tests, ESwab® gives flexibility and increased performance as compared to other collection devices
ESwab® 480C

with Liquid Amies
Medium
ESwab® 481C

with Liquid Amies
Medium
ESwab® 482C

Flocked Swab
with Liquid Amies Medium
Stay Informed
With tens of thousands of scientific papers published each year, it is hard to stay informed on the relevant research. Each month COPAN’s Research Roundup will feature a comprehensive review and commentary on recent scientific publications covering a particular topic by experts in the field to help keep you up to date on the latest research. Sign up today so you don’t miss the latest findings!
About Dr. Susan Sharp

Dr. Sharp has been a clinical microbiologist and active member of the American Society for Microbiology (ASM) for 30 years serving on various boards and committees. She is a Diplomat of the American Board of Medical Microbiology (ABMM) and a Fellow in the American Academy of Microbiology. Dr. Sharp served as the Chair of the ASM Public and Scientific Affairs Board’s Committee on Laboratory Practices from 2007 to 2015, as well as Chair of the Examination Development committee and Vice-Chair of the ABMM during 1999-2015.
Dr. Sharp has recently served as an Advisor to the CLSI Antimicrobial Susceptibility Testing Sub-Committee and she is a current member of the CLSI AST Resistance Working Group as well as the Methods Application and Interpretation Working Group. She is also an active member of the Board of Scientific Councilors for the Office of Infectious Diseases of the Centers for Disease Control and Prevention. Dr. Sharp is a Past-President of ASM and a former member of ASM’s Board of Directors. Dr. Sharp has given numerous lectures, seminars and workshops locally, nationally and internationally, and has numerous publications in the field of clinical microbiology. Her most prominent area of interest has centered on cost-effective, clinically-relevant diagnostic microbiology.

