PhenoMATRIX® Receives FDA 510(k) Clearance: Advancing Digital Microbiology with Confidence
 Read More
Read More

For clinical microbiology laboratories navigating increasing workload demands, staffing pressures, and the need for standardized processes, this milestone represents more than regulatory achievement; it represents progress.
February 12, 2026

The investment includes facility expansions, new manufacturing processes, advanced quality control systems, and implementation of new production technologies in the U.S. This initiative accelerates production of critical components and finished goods, such as UTM® Universal Transport Medium kits, used for collecting respiratory viral specimens, and UriSponge®, Copan’s novel urine collection and transport device.
September 24, 2025

Copan Diagnostics announced the appointment of Dr. Hema Kapoor as Director of Medical and Scientific Affairs for the Americas, succeeding Dr. Susan Sharp, who will retire in September following more than three decades of distinguished service to clinical microbiology.
August 27, 2025

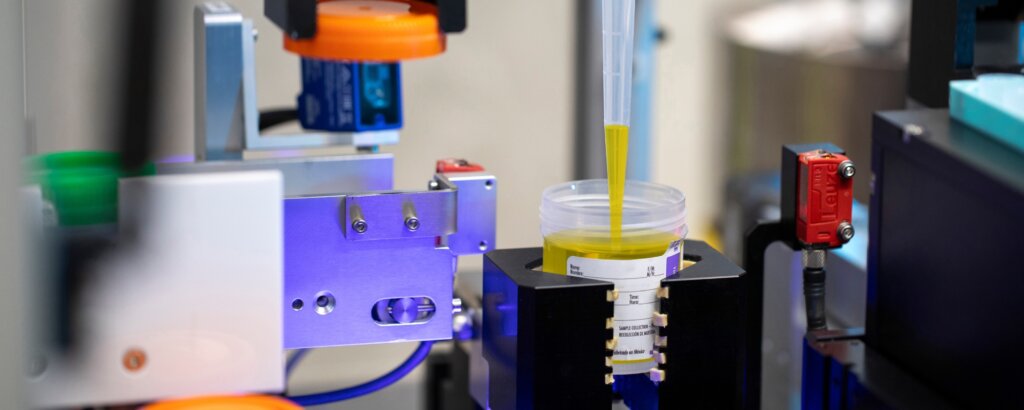
Copan, a leader in microbiology laboratory and automation innovations, proudly announced the official launch of UriVerse™ during the Association for Diagnostics & Laboratory Medicine (ADLM) Clinical Lab Expo in Chicago, IL.
August 12, 2025

Hospital Angeles Pedregal has completed the first installation of Copan's WASPLab® automated system in Latin America, marking a milestone for laboratory medicine in the region. Complete Digital Microbiology: The system provides comprehensive automation from specimen processing to smart incubation, digital imaging, and enhanced workup management, revolutionizing traditional laboratory workflow. Workflow Optimization: WASPLab® seamlessly links specimen processing, incubation, imaging, analysis, and result output – eliminating manual workflow practices. Estimated Reading Time: 8 Minutes
February 25, 2025