PodSwab™ FAQ
This FAQ covers key questions about how PodSwab™ works and the science behind pooled testing. We explain what PodSwab™ is, how sample pooling allows efficient use of tests, directions for use, and how to set up a program.
What is a PodSwab™?
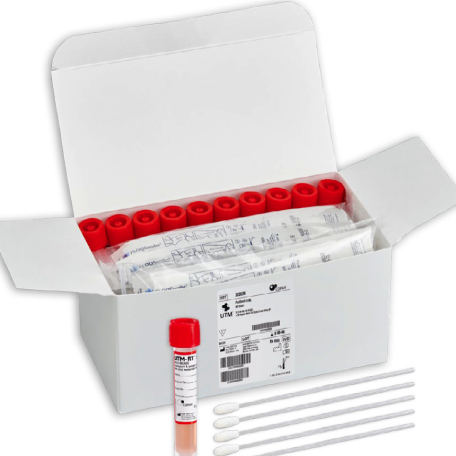
Designed to streamline and ease the burden of COVID-19 testing, PodSwab™ consists of 6 mL UTM® Universal Transport Media™ and 5 nasal flocked swabs allowing for up to five different individual samples in each tube. This format is designed for testing groups or pods of people, helping businesses and schools efficiently screen employees and students, saving costs and time. This efficient grouped method of testing is called pooled testing.
What is UTM® Universal Transport Media™?
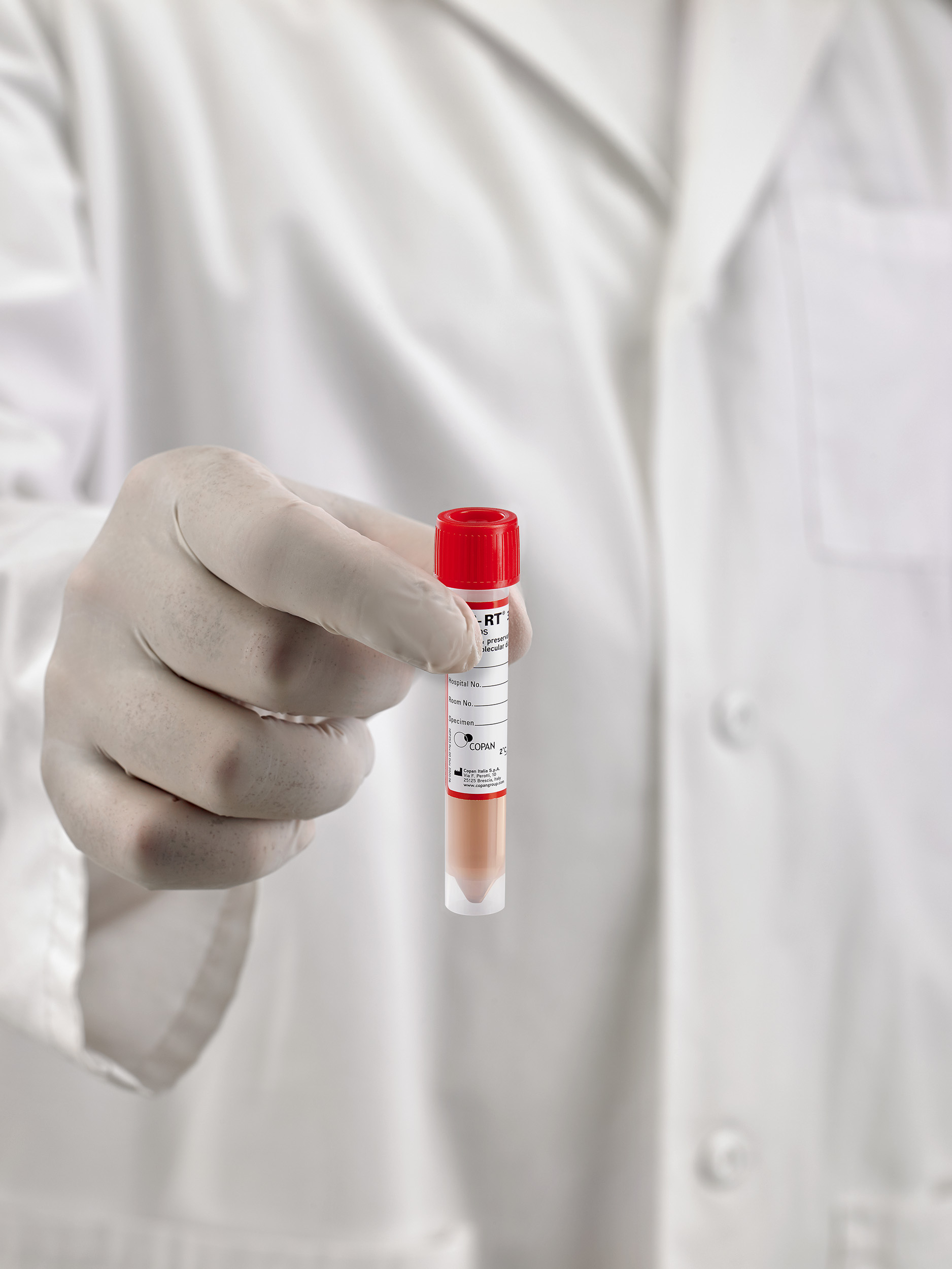
UTM® Universal Transport Media™ is an FDA-cleared collection and transport system suitable for collection, transport, maintenance, and long-term freeze storage of clinical specimens containing viruses, including COVID-19. The transport medium-filled tube, complete with plastic screw cap, and keeps organisms viable for 48 hours at room or refrigerated temperature.
How Does Sample Pooling Work?
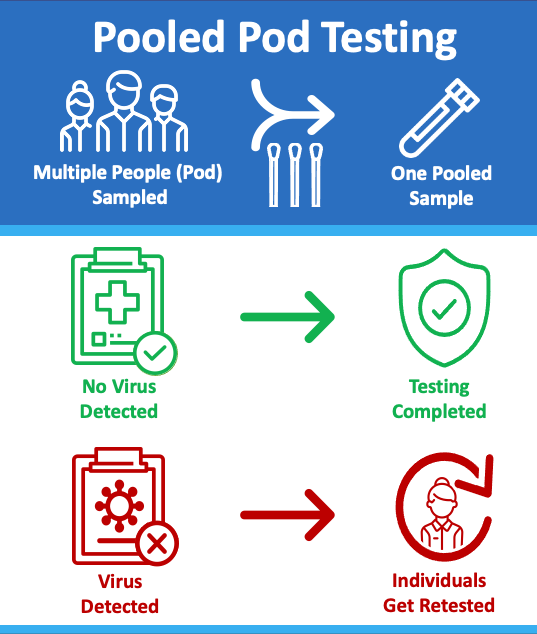
SAMPLE POOLING or Pod Testing is a cost-effective method for screening groups where the expected positivity rate is low.
The concept for pooling samples looks like this:
- Samples are collected from a group, or pod of people using one swab per person.
- The samples are then combined together into a single tube.
- If the pooled test is negative for COVID, then all people in the pod are considered negative.
- If a pooled test result is positive, then each person within the pod must be individually retested to determine who was positive.
Is COVID-19 Sample Pooling Backed by Science?
The concept of pooled testing isn’t a novel one— the United States first tried the method out during World War II to screen soldiers for syphilis. It’s an effective tool that not only preserves testing supplies but also saves time.
Pooling COVID sample is backed by scientific studies and the FDA has provided resources for developers and laboratories that would like to better manage resources using pooled testing (Pooled Sample Testing and Screening Testing for COVID-19 | FDA).
Does COPAN Have Other COVID-19 Related Products and Information?
Yes, COPAN Provides numerous products for the Collection and Transport of COVID Samples for the Healthcare, Government, and Laboratory sectors sold through distributors. COPAN has compiled a COVID-19 resource page to facilitate answers to some of the frequently asked questions. Here you’ll find easy access to the evolving regulatory guidelines published by the CDC and FDA, COPAN’s distributor part numbers, and other resources about critical products that can be used for collecting, handling, and transporting specimens suspected of COVID-19.

