Back to Work Sample Collection for COVID-19 Pooled Samples
March 24, 2021
As the COVID testing positivity rate has declined, new, more efficient, and less expensive COVID testing strategies have been developed. The adoption of ‘pooled’ strategies allows for significant cost savings in sample collection and testing supplies, especially in populations with low positivity rates. With pooled testing, more individuals can be screened with fewer resources. And as children start streaming back into the classroom and employees back to the office, screening large numbers of asymptomatic populations for the SARS-CoV-2 virus has become more critical. Those in charge of mass testing are looking at ways to make the job less burdensome while maintaining quality and improving the turnaround time.
What is a Pooled COVID Testing Strategy?
The concept of pooled testing isn’t a novel one— the United States first tried the method out during World War II to screen soldiers for syphilis. It’s an effective tool that not only preserves testing supplies but also saves time.
The concept for pooling samples looks like this: samples are collected from a group, or pod of people (usually around 3-10 individuals) using one swab per person. The samples are then combined together into a single tube. This can be done at the sample collection point by combining multiple swabs into one collection tube. Or at the laboratory level by combining a predetermined amount of different patients’ specimens into one test tube. If the pooled test is negative for COVID, then all people in the pod are considered negative. If a pooled test result is positive, then each person within the pod must be individually retested to determine who was positive.
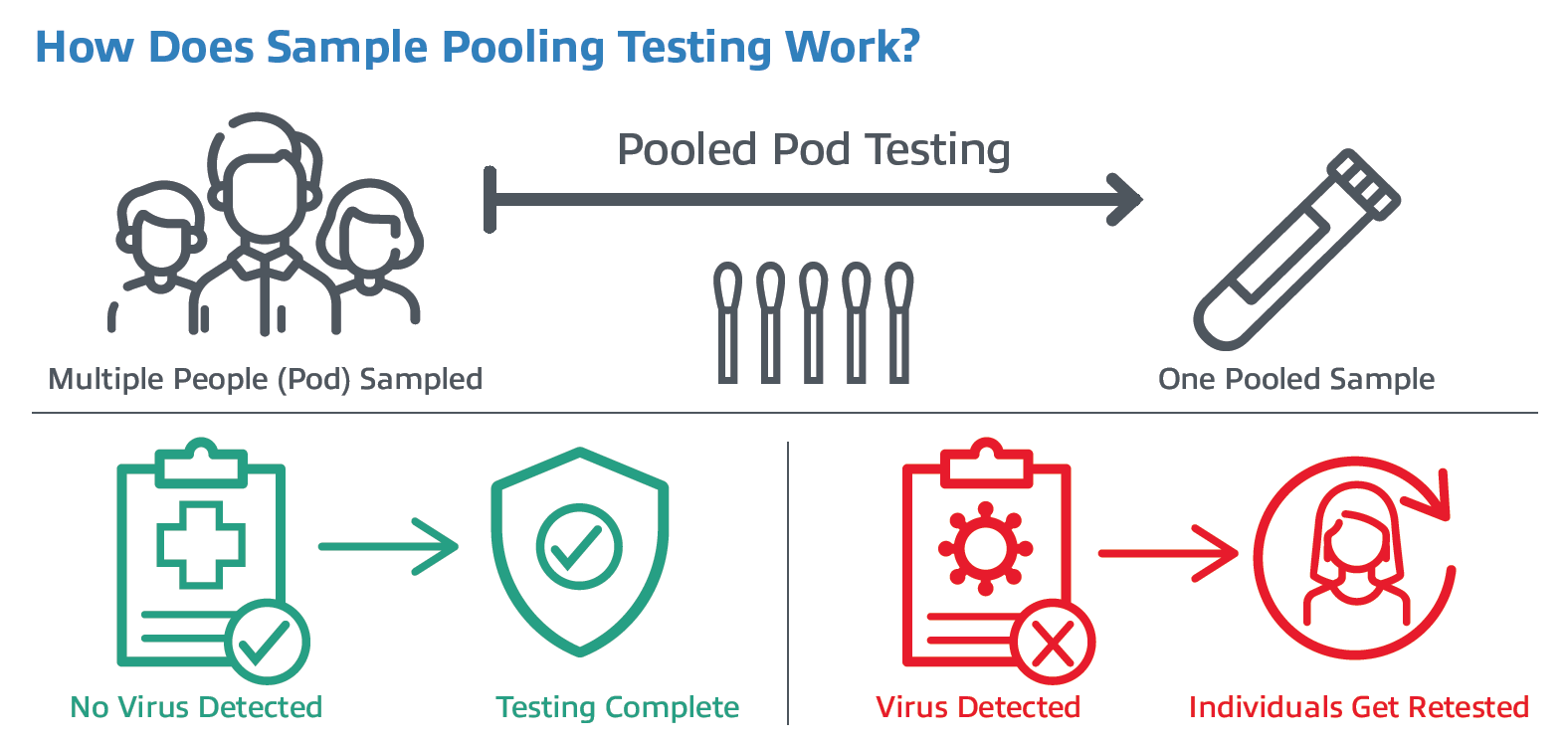
There are many examples of swab pooling in current practice around the world in K-12 schools and colleges. In many of these efforts, pods of students and staff are tested via pooling from on-site tests with medical personnel or using at-home COVID-19 tests. If the pod tests negative, then all students/staff in that pod are negative, and lessons continue.
However, if the pod tests are positive, the class is closed, and students are sent home with instructions to get tested as soon as possible, or the school may elect to retest the students individually on-site. This type of pod testing can be repeated at regular intervals, confident that students and staff are safe while learning and teaching in person. In the end, the researchers concluded that “swab pooling represents a major alternative for reliable and large-scale screening of SARS-CoV-2 in low prevalence populations.”
By pooling samples, testers are able to significantly increase the number of individuals that can be tested at a lower cost. Pooling increases the number of individuals that can be tested using the same amount of resources and can detect both symptomatic and asymptomatic persons.
Pooling Strategies Approved by the FDA
The FDA has provided resources for developers and laboratories that would like to better manage resources using pooled testing (Pooled Sample Testing and Screening Testing for COVID-19 | FDA). They offer two types of pooling strategies:
Sample/Media pooling: Pooling together testing transport media, each containing one patient specimenb)
Swab pooling: Adding swabs from multiple patients into a single volume of transport media
The FDA recommends that pooling strategies have a test performance of ≥ 85% positive agreement (PPA) when compared with the same test performed on individual samples. It is also important to note that reporting a negative pool result should indicate that the testing procedure involved specimen pooling and explain the limitations of that type of testing.
Sample/media pooling, where aliquots of transport media are pooled into a single sample for testing, conserves laboratory testing materials but not collection device swabs and media. On the other hand, swab pooling will save on both laboratory testing materials and collection device materials. With issues surrounding the supply chain of both testing supplies and collection devices, pooling has proven an advantageous way to obtain testing results with a high level of sensitivity.
Enter PodSwab™, a New Tool for Testing Groups for COVID-19

With pooled testing, more individuals can be screened using fewer resources . As back to work and back to school testing programs are deployed across the US, it becomes critical to have the correct tools in place to screen large numbers of asymptomatic populations for SARS-CoV-2.
That’s why Copan is retooling UTM® tubes by adding a few extra milliliters of medium and packaging them with 5 flocked swabs to create PodSwab™. In order to test pods in schools and workplaces, the new PodSwab™ with 6 mL UTM® was explicitly designed to streamline and ease the burden of COVID-19 testing.
UTM®: Universal Transport Medium™ is an FDA-cleared collection and transport system suitable for collection, transport, maintenance, and long-term freeze storage of clinical specimens containing viruses, including COVID-19. The transport medium-filled tube, complete with plastic screw cap, maintains organism viability for 48 hours at room or refrigerated temperature.
Each PodSwab™ consists of a 6 mL UTM®: Universal Transport Medium™ tube and five nasal flocked swabs allowing for up to five different individual samples in each tube.
This is welcome news in the face of skyrocketing Coronavirus screening and surveillance tests. And considering that post-pandemic life may continue to include regular testing — schools, airlines, sporting events etc. — PodSwab™ will help reduce the testing workload while still maintaining the product’s trusted results.
How PodSwab™ Sample Collection Works
Pooling Techniques in Scientific Literature
Sample/Media Pooling:
One example of sample/media pooling is in the publication by Borillo et al, on PubMed from (nih.gov) where testing accuracy was assessed by pooling positive specimens with negative specimens. Both pooled and individual tests of specimens positive for SARS-CoV-2 showed 100% agreement demonstrating that pooled specimen testing can improve testing capacity while reducing reagent and supply utilization. This system affords better access and a more rapid turnaround time for patient results (1).
Likewise, a paper by Sawicki et al (nih.gov), demonstrated that sample/media pool testing could detect up to a single positive sample with Ct value as high as 34 in a pool of 16 specimens resulting in saving 96 tests (2).
Swab Pooling:
A publication by Christoff et al, at (plos.org) evaluated the effectiveness of swab pooling at the time of collection as opposed to pooling of equal volumes from individually collected. Paired analysis of pooled (16 persons to a pool) and individual samples from 613 patients revealed 94 positive individuals. No false positives or false-negatives were observed for swab pooling. A total of 19,535 asymptomatic patients were screened in the study. The results? In addition to minimizing sample dilution, the pooling process corresponded to an increase of 4.4 times in laboratory capacity and a significant reduction in the lab’s workload since 77 percent fewer tests were required.
In the end, the researchers concluded that “swab pooling represents a major alternative for reliable and large-scale screening of SARS-CoV-2 in low prevalence populations” (3).
What is PodSwab™?
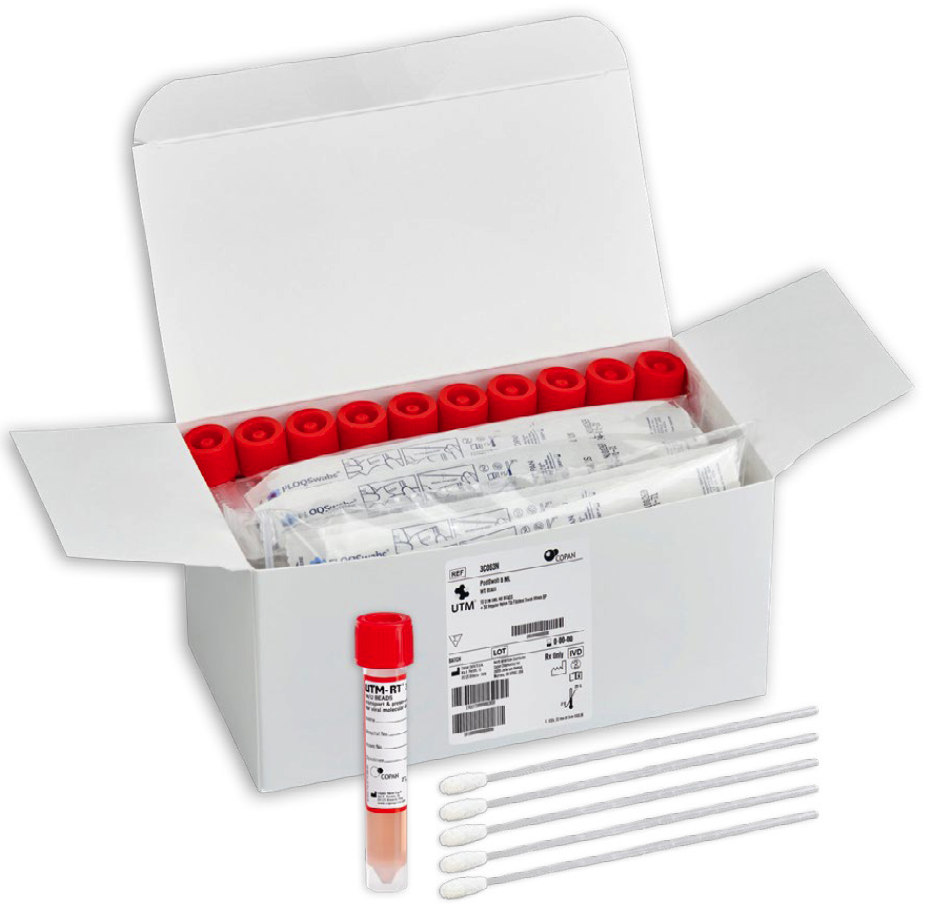
Designed to streamline and ease the burden of COVID-19 testing, PodSwab™ consists of 6 mL UTM®: Universal Transport Medium™ and 5 nasal flocked swabs allowing for up to five different individual samples in each tube. This format is designed for testing groups or pods of people, helping businesses and schools efficiently screen employees and students, saving costs and time. This efficient grouped method of testing is called pooled testing.
COVID-19 Resource Page

COPAN has compiled a COVID-19 resource page to facilitate answers to some of the frequently asked questions being received. Here you’ll find easy access to the evolving regulatory guidelines published by the CDC and FDA, COPAN’s distributor part numbers, and other resources about critical products that can be used for collecting, handling, and transporting specimens suspected of COVID-19.
Click Here to Go to the COVID-19 Resource Page for Coronavirus Products and Resources
References:
1. Borillo GA, Kagan RM, Baumann RE, Fainstein BM, Umaru L, Li HR, Kaufman HW, Clarke NJ, Marlowe EM. Pooling of Upper Respiratory Specimens Using a SARS-CoV-2 Real-time RT-PCR Assay Authorized for Emergency Use in Low-Prevalence Populations for High-Throughput Testing. Open Forum Infect Dis. 2020 Sep 29;7(11):ofaa466. doi: 10.1093/ofid/ofaa466. PMID: 33204756; PMCID: PMC7543567.
2. Sawicki R, Korona-Glowniak I, Boguszewska A, Stec A, Polz-Dacewicz M. Sample pooling as a strategy for community monitoring for SARS-CoV-2. Sci Rep. 2021 Feb 4;11(1):3122. doi: 10.1038/s41598-021-82765-5. PMID: 33542424; PMCID: PMC7862381.
3. Christoff AP, Cruz GNF, Sereia AFR, Boberg DR, de Bastiani DC, Yamanaka LE, et al. (2021) Swab pooling: A new method for large-scale RT-qPCR screening of SARS-CoV-2 avoiding sample dilution. PLoS ONE 16(2): e0246544. https://doi.org/10.1371/journal.pone.0246544