UTM® FAQ
This FAQ page provides information on UTM®, a viral transport medium. Topics covered include what UTM® is, how it is used, what is in the formulation, viability duration, and availability.
Contents
- What is UTM®: Viral Transport Medium?
- How is UTM® Used?
- Illustrated UTM® Sampling Guides
- UTM® How-To Video
- What is In UTM® Viral Transport Medium?
- How Long Does the UTM®: Viral Transport Medium Maintain the Viability of the Specimen?
- Can UTM®: UTM®: Universal Transport Medium™ Be Used For Molecular or Antigen Testing?
- Can UTM®: Universal Transport Medium™ Be Used For Flu Testing?
- Can UTM® Be Used for COVID-19 Testing?
- Is UTM®: Viral Transport Medium Available in The United States?
- Online Ordering and Distributor Links
- Do You Have Other Questions about UTM® FAQ?
What is UTM®: Viral Transport Medium?
UTM® is a FDA cleared collection and transport system suitable for collection, transport, preservation, and long-term freeze storage of clinical specimens containing viruses for viral molecular diagnostic testing, including COVID-19, chlamydia, mycoplasma or ureaplasma organisms and meets CDC Interim Guidelines for Collecting, Handling, and Testing Clinical COVID-19 Specimens.
UTM® Universal Transport Medium™‘s unique media formulation includes antibiotics to inhibit bacterial and fungal growth, without affecting viruses. The transport medium comes in a plastic, red screw cap tube. The transport medium comes in a plastic screw cap tube and maintains organism viability for 48 hours at room or refrigerated temperature.
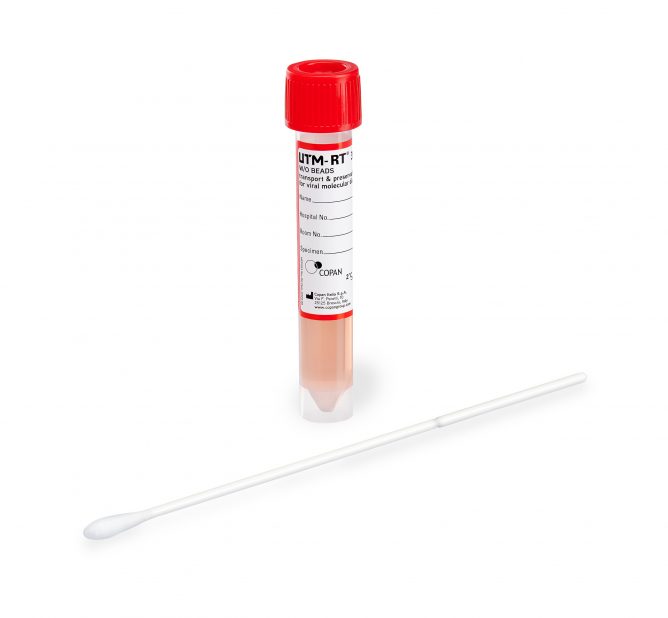
How is UTM® Used?
Using the swab provided in the collection kit, a sample can be taken at the appropriate sampling site and transferred directly into the tube. For respiratory diseases like COVID-19 or Influenza, the following testing sites are recommended**:
- Oropharyngeal specimen using a flocked swab
- Mid-turbinate specimen using a flocked tapered swab
- Anterior nares specimen using a round foam or polyester swabs
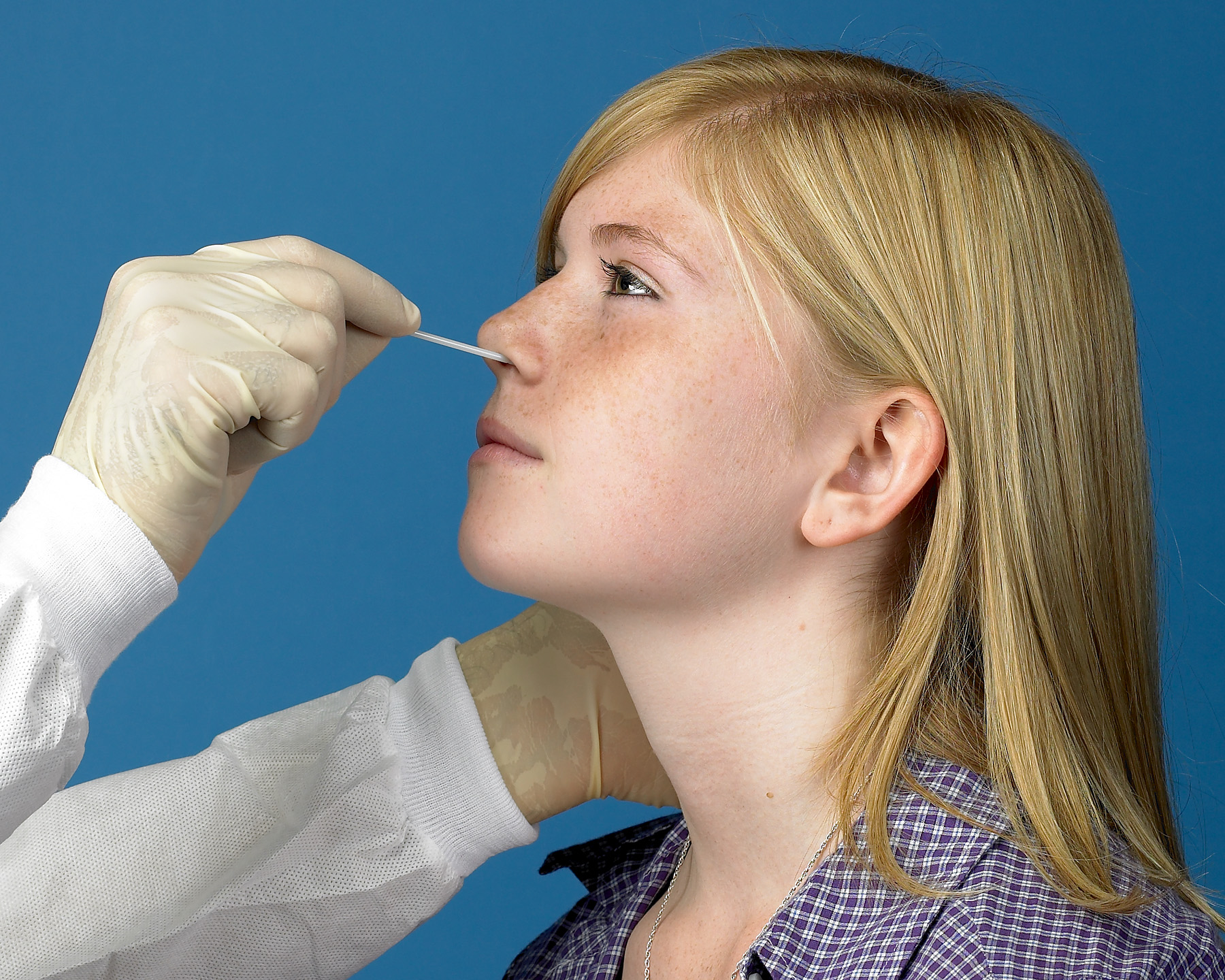
Illustrated UTM® Sampling Guides
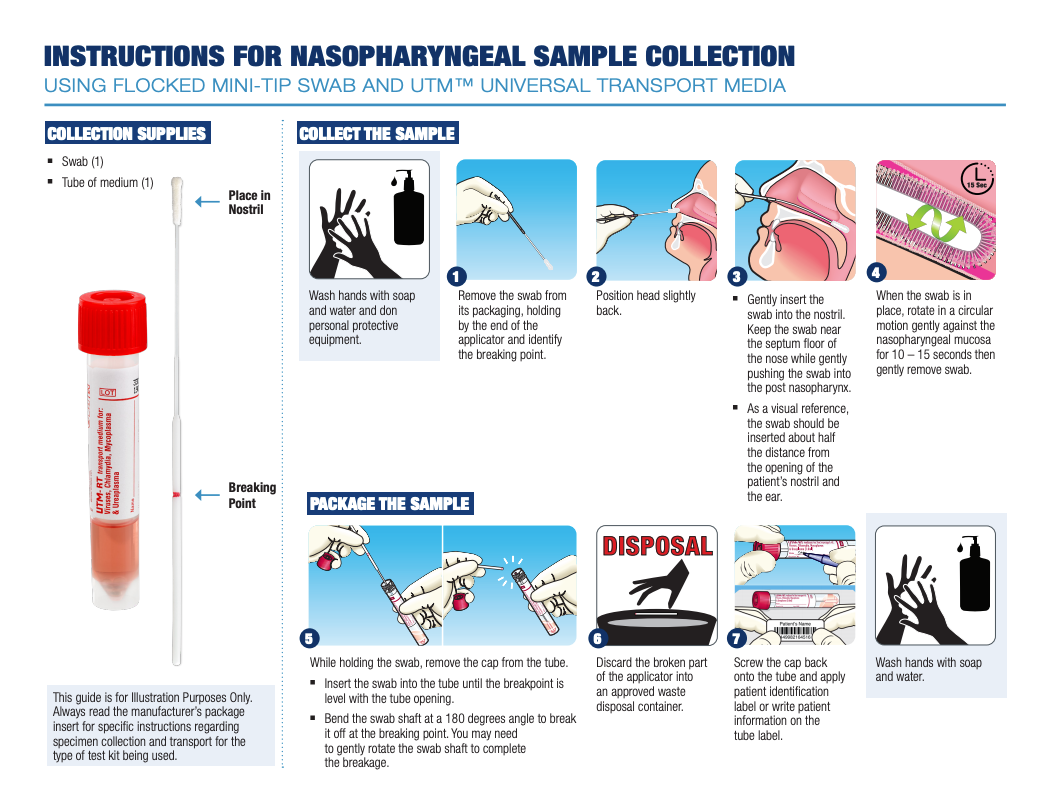
Below are examples of guides that can be used to illustrate how to collect viral specimens, like COVID-19 or influenza, from different sites. Always read the manufacturer’s package insert for specific instructions regarding specimen collection and transport for the type of test kit being used.
Nasopharyngeal UTM® Collection Guide
Oropharyngeal UTM® Collection Guide
UTM® How-To Video
One of the most widely used methods for detection of influenza viruses is to collect a nasopharyngeal swab sample. A patient collection pack comprising COPAN UTM and Flocked Swabs is ideal to collect and transport such samples. Click to watch this video on the proper method to collect a nasopharyngeal sample using a COPAN patient collection pack.
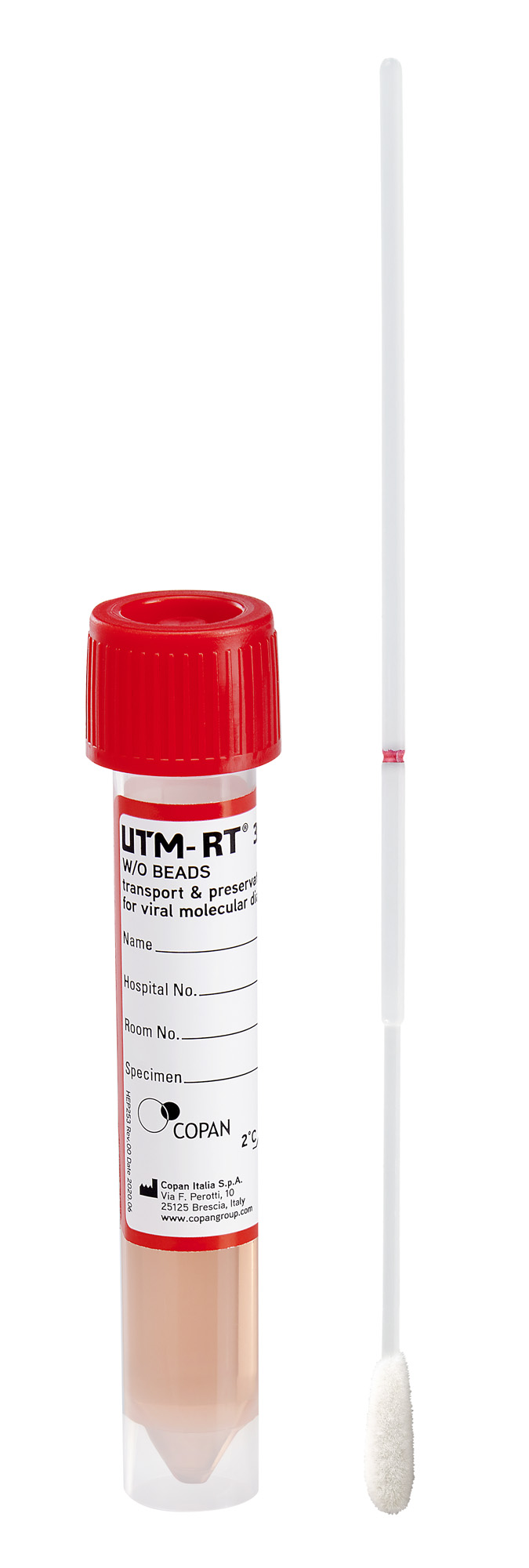
What is In UTM® Viral Transport Medium?
The UTM® Viral Transport Medium formulation includes proteins for virus stabilization, antibiotics, and antimycotics to prevent overgrowth of bacterial and fungal flora and a buffer solution to maintain a neutral pH. A phenol red pH indicator provides a visual gauge of the medium integrity throughout the storage and life of the product.
How Long Does the UTM®: Viral Transport Medium Maintain the Viability of the Specimen?
- UTM® is a FDA cleared collection and transport system suitable for collection, transport, preservation, and long-term freeze storage of clinical specimens containing viruses for viral molecular diagnostic testing, including COVID-19, chlamydia, mycoplasma or ureaplasma organisms and meets CDC Interim Guidelines for Collecting, Handling, and Testing Clinical COVID-19 Specimens.
- UTM® Universal Transport Medium™’s unique media formulation includes antibiotics to inhibit bacterial and fungal growth, without affecting viruses. The transport medium comes in a plastic, red screw cap tube. The transport medium comes in a plastic screw cap tube and maintains organism viability for 48 hours at room or refrigerated temperature.
Can UTM®: UTM®: Universal Transport Medium™ Be Used For Molecular or Antigen Testing?
UTM® has been used successfully for Rapid Antigen Testing, DFA, and for Molecular-Based Assays * Unique media formulation includes antibiotics to inhibit bacterial and fungal growth, without affecting viruses, chlamydia, mycoplasma or ureaplasma
Can UTM®: Universal Transport Medium™ Be Used For Flu Testing?
Yes, UTM® is suitable for the collection, transport, and maintenance of influenza specimens.

Can UTM® Be Used for COVID-19 Testing?
Yes, UTM® is suitable for collection transport and maintenance for COVID-19 specimens. COPAN has compiled a COVID-19 resource page to facilitate answers to some of the frequently asked questions being received. Here you’ll find easy access to the evolving regulatory guidelines published by the CDC and FDA, COPAN’s distributor part numbers, and other resources about critical products that can be used for collecting, handling, and transporting specimens suspected of COVID-19.

Is UTM®: Viral Transport Medium Available in The United States?
Yes, UTM® received FDA Clearance and, UTM® has been tested and validated in full compliance with CLSI M40-A2: Quality Control of Microbiological Transport System Standard.
Online Ordering and Distributor Links
COPAN now offers purchasing sample collection supplies directly from the leading manufacturer in sample collection! Click the button below to visit our new online storefront. Our products are also available through our distribution partners.
*Always read the manufacturer’s package insert for specific instructions regarding specimen collection and transport for the type of test kit being used.
** Check for current guidance with the FDA, CDC, or other local, state, or federal governing bodies for the latest recommendations.


