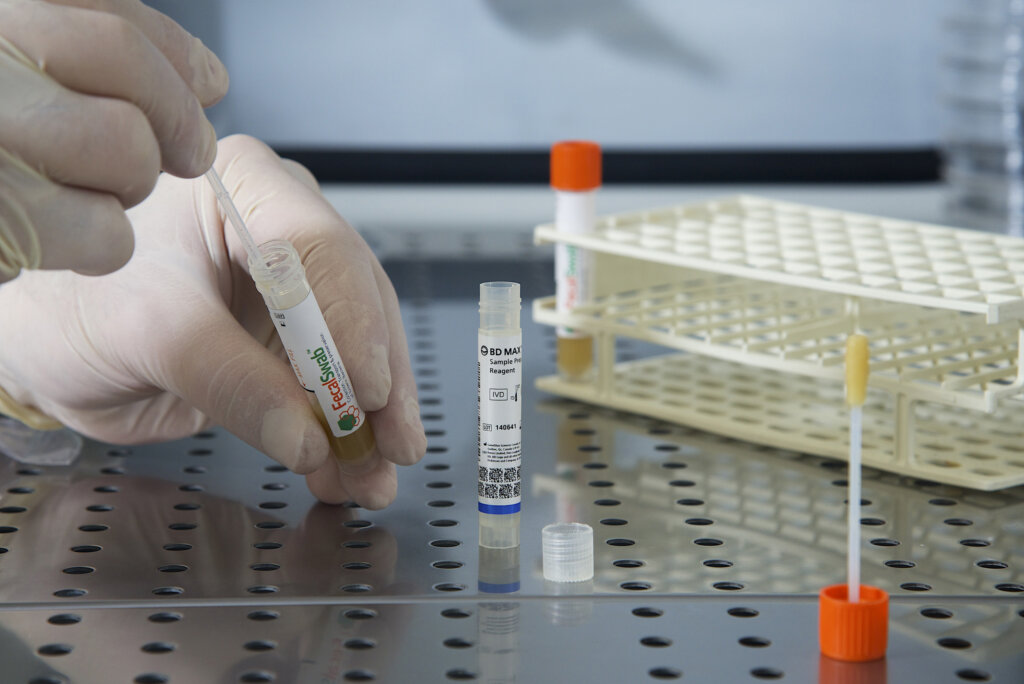
Study Validates Effectiveness of Copan FecalSwab® for Enteric Pathogen Detection on the BD MAX™ System
 Read More
Read More
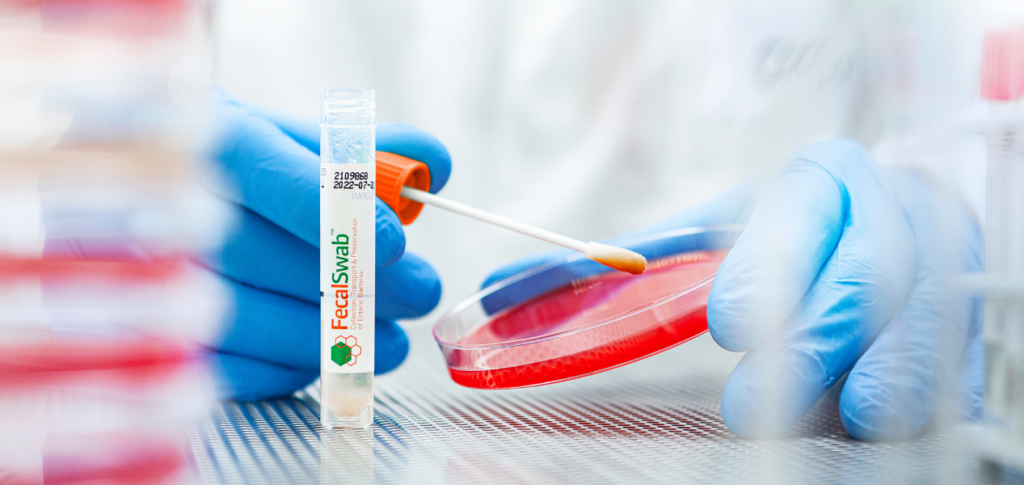
Comprehensive Guide to Cary-Blair Transport Medium: The Gold Standard for Fecal Sample Collection and Transport
 Read More
Read More
Study Presented at CACMID 2024 Highlights Copan's UniVerse® as a Game-Changer in Clinical Molecular Specimen Preparation
 Read More
Read More
Study Demonstrates Effectiveness of eNAT® Medium for Long-Term Fecal Microbiome Sample Preservation
 Read More
Read More
New Study Shows Copan's Colibrí™ Automates MALDI-TOF Sample Preparation with High Accuracy
 Read More
Read More
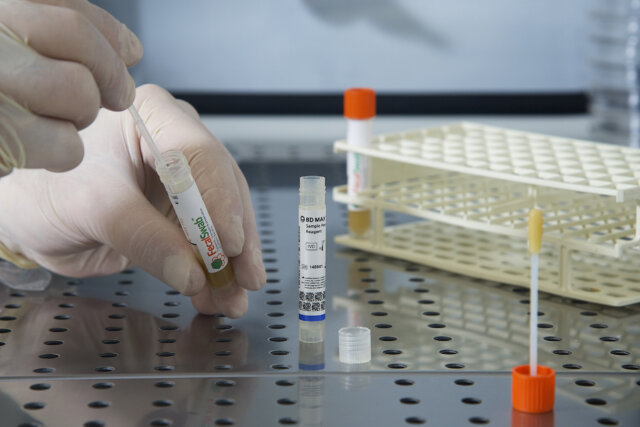
Researchers from McMaster University and St. Joseph's Healthcare validate Copan’s FecalSwab® system with the BD MAX™ platform for detecting enteric pathogens.
August 26, 2024
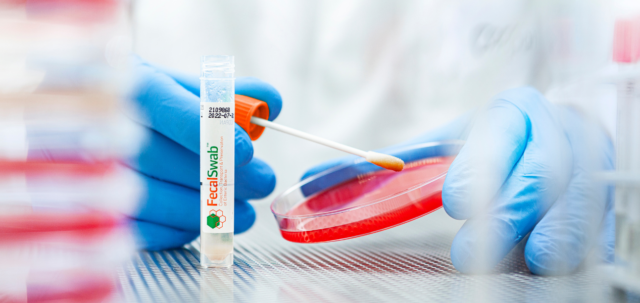
This article details the specific components of Cary-Blair medium and their functions, highlighting its effectiveness in preserving a wide range of enteric pathogens during transport from collection sites to laboratories.
August 23, 2024

Study Presented at CACMID 2024 Highlights Copan's UniVerse® as a Game-Changer in Clinical Molecular Specimen Preparation
July 22, 2024

Study Demonstrates Effectiveness of eNAT® Medium for Long-Term Fecal Microbiome Sample Preservation
May 7, 2024

New Study Shows Copan's Colibrí™ Automates MALDI-TOF Sample Preparation with High Accuracy
May 1, 2024

Embracing the Future of Clinical Microbiology: A Deep Dive into Laboratory Automation
July 21, 2023

This study highlights the effectiveness of The SnotBuster™ (also known as SLSolution™) in processing lower respiratory tract specimens. It enables superior bacterial detection and allows for faster work-up of more pathogens compared to traditional methods. The system improves efficiency in both manual and automated labs, offering clearer Gram stain results and better specimen handling.
October 24, 2022

