Collection and Preservation System for Molecular Assays and Gram-Positive Bacteria
MSwab® is a collection, transport and preservation system designed for rapid direct nucleic acid heat extraction for real-time PCR and isothermal amplification for molecular assays.
MSwab® is intended for the collection and transport of clinical specimens containing:
For the following downstream applications:
MSwab® is not suitable for:
MSwab® has the advantage of enabling reflex culture for traditional culture of gram-positive aerobic and facultative anaerobic bacteria.

MSwab® generates crude lysates in under 5 minutes, enabling direct heat extraction of nucleic acids while eliminating lengthy purification steps.
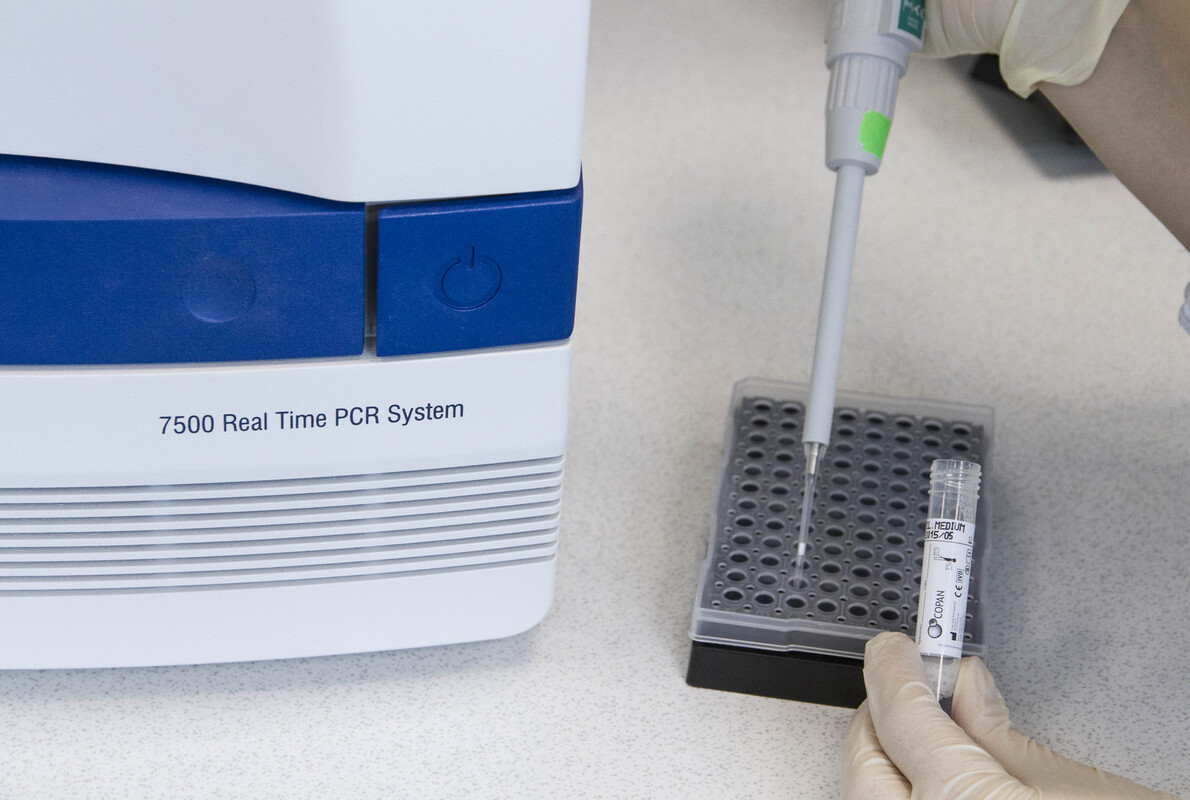
Post-extraction, MSwab® specimens aliquot directly into Master Mixes, making direct molecular assays even faster.

MSwab® media is developed to prevent RNA/DNA degradation, preserving samples for PCR and molecular assays for bacterial or viral investigations.
Ensure a quick, capillarity-driven sample uptake and a superior elution of the biological specimen, expanding downstream diagnostic testing capabilities.
MSwab® enables bacterial culture of aerobic and facultative anaerobic gram-positive microorganisms, viral culture of HSV1/2, and viral/bacterial nucleic acid detection using standard clinical procedures.

MSwab® pairs COPAN's patented FLOQSwabs® flocked collection swabs with a proprietary media developed to prevent RNA/DNA degradation of samples for PCR and molecular assays. MSwab™ is qualified for the Roche cobas® 4800 System for MRSA/SA and HSV 1 & 2.
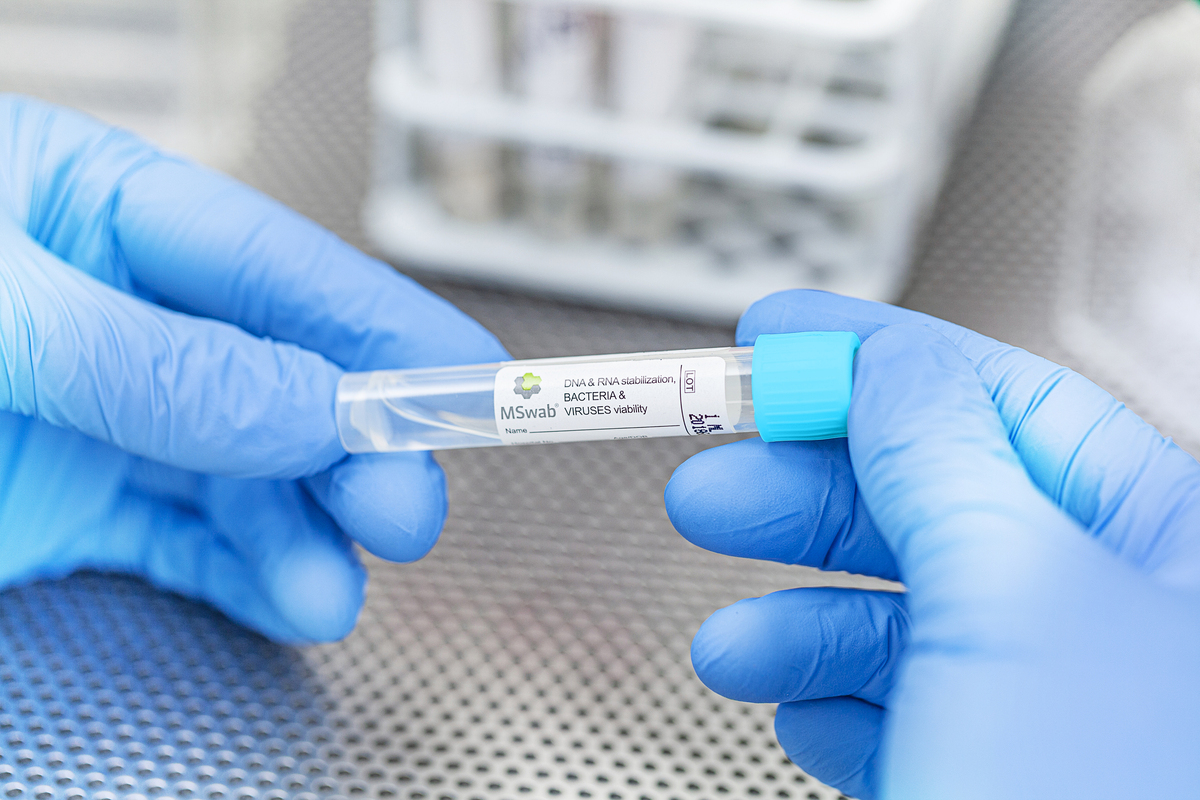
MSwab® medium homogeneously suspends specimens, enabling multiple assays from a single sample aliquot. Combined with Copan's patented FLOQSwabs® for complete elution, MSwab® creates a versatile testing solution from one tube.
MSwab® is a collection, transport and preservation system designed for rapid direct nucleic acid heat extraction for real-time PCR and isothermal amplification for molecular assays.
MSwab® is intended for the collection and transport of clinical specimens containing:
For the following downstream applications:
MSwab® is not suitable for:
There are no results matching your query.
Swab: Nylon® Flocked Swab
Tip: Regular
Breakpoint: 80mm
Tube: 12x80mm
Cap: Blue Capture
Individually Packaged, Sterile 50/Pack; 6 Packs/Case
Copan specializes in working with partners in assay manufacturing, microbiome research, and at home collection kit developers and more to create customized swabs and kits, including filling of tubes with custom reagents. Contact us today to speak to a knowledgeable Copan team member.