eNAT® FAQ
This FAQ page provides information on eNAT®, a sample collection and transport system that stabilizes and preserves nucleic acids for molecular testing, covering topics such as what eNAT® is, how it works, applications, compatibility, and availability.
Contents
- What is eNAT®?
- What kind of preservation medium is in eNat® and how is eNat® used?
- Is eNat® FDA-Cleared?
- Where can I learn more about eNat®?
- How long does eNat® stabilize a molecular sample?
- Does eNat® medium inactivate nucleases?
- Is eNat® suitable for remote testing without a consistent cold-chain?
- How are eNat® samples collected?
- Is eNat® compatible with my laboratory’s molecular platform?
- Is eNat® compatible with Cepheid Xpert Xpress Flu/RSV Assay?
- Can I use eNat® with Master Mix?
- Can eNat® be processed using COPAN UniVerse®?
- Is eNat® safe to use with potentially infectious specimens?
- Is eNat® suitable for rapid antigen testing or cell culture?
- Can eNat® be used for COVID-19 testing?
- Where can I purchase eNat®?
- Do You Have Other Questions about eNAT® FAQ?
What is eNAT®?
COPAN’s eNat® system is intended for the collection and preservation of viruses, including upper respiratory tract specimens, bacteria, Chlamydia, Trichomonas vaginalis, and Mycoplasma. The sample collection system completely stabilizes and renders the specimen non-infectious within minutes. eNat® is a versatile system combining a COPAN flocked swab with a guanidine-thiocyanate based medium to stabilize RNA and DNA of viruses and bacteria.

What kind of preservation medium is in eNat® and how is eNat® used?
eNat® medium is a versatile molecular medium specially designed to stabilize and preserve microbial nucleic acids (RNA/DNA) for prolonged time periods. eNat® securely inactivates specimens that may contain highly infectious agents, such as SARS-CoV-2 (the virus which causes COVID-19), using a detergent and a protein denaturant to completely neutralize microbial viability within minutes. Nuclease inactivation provided by eNat® ensures the integrity of the target sequences while protecting polynucleotides against exposure to DNases and RNases. Specimens preserved in eNat® can be stored for up to four weeks at room temperature or 4°C or at -20°C for up to six months.
Is eNat® FDA-Cleared?
Yes, eNat® has been FDA cleared for use in the detection of influenza A viruses (REF: FDA K201849). The sample collection and transport system also has implied use for specimens suspected of containing other viruses, including SARS-CoV-2.
Where can I learn more about eNat®?
eNat® was recently featured in COPAN’s Research Roundup, where Dr. Susan Sharp gives a comprehensive review and commentary on recent scientific publications covering the use of eNat® for COVID-19 sample collection.
You can also search our Scientific studies section to find related research articles about eNat™ and any COPAN product.

How long does eNat® stabilize a molecular sample?
eNat® is a versatile molecular medium specially designed to stabilize and preserve microbial (RNA/DNA) for prolonged time periods to be used for future testing at room temperature for up to four weeks or at -20°C for up to six months.
Does eNat® medium inactivate nucleases?
Yes. Nuclease inactivation provided by the eNat® medium ensures the integrity of the target sequence and protects polynucleotides against exposure to DNases and RNases.
Is eNat® suitable for remote testing without a consistent cold-chain?
Yes. A recent study by Richard-Greenblatt et al., 2021, evaluated the use of eNat® for the collection of samples suspected of containing SARS-CoV-2. The study demonstrates that, when the virus was placed into eNat® solution, it was rendered inactive as it could no longer be grown in viral culture. The study went on to show that the specimen was stable for up to 14 days at a wide temperature range (4°C-35°C). The study concluded that eNat® has the potential to expand COVID-19 testing to areas with limited healthcare access without the worry of cold-chain requirements or biosafety concerns.

How are eNat® samples collected?
Accurate test results depend on proper specimen collection, transport, storage, and processing in the laboratory. Specimens should be collected by qualified medical personnel. Follow biohazard precautions and aseptic techniques when collecting specimens. For specific guidance regarding specimen collection procedures, consult published collection manuals.*
*Always read the manufacturer’s package insert for specific instructions regarding specimen collection and transport for the type of test kit being used.

Is eNat® compatible with my laboratory’s molecular platform?
eNat® is compatible with many molecular platforms and methods with prior validation. Always read the manufacturer’s package insert for specific instructions regarding specimen collection and transport for the type of test kit being used.
- IMPORTANT NOTE: eNat® medium is not compatible with instruments that use bleach disinfection, such as the Hologic Panther® or Panther Fusion® systems. If the laboratory deactivates the disinfecting step involving bleach specimens collected in eNat™ can be used.
Is eNat® compatible with Cepheid Xpert Xpress Flu/RSV Assay?
Yes, COPAN eNAT® is specifically indicated as a suitable sample collection media for nasopharyngeal and anterior nasal swab samples for the Cepheid Xpert® Xpress Flu/RSV Assay. eNAT® may also be compatible with other molecular platforms as indicated by the manufacturer’s package insert or with prior validation.

Can I use eNat® with Master Mix?
Yes, but nucleic acids must be extracted from eNat® and purified prior to adding to the Master Mix.*
*Always read the manufacturer’s package insert for specific instructions regarding specimen collection and transport for the type of test kit being used.
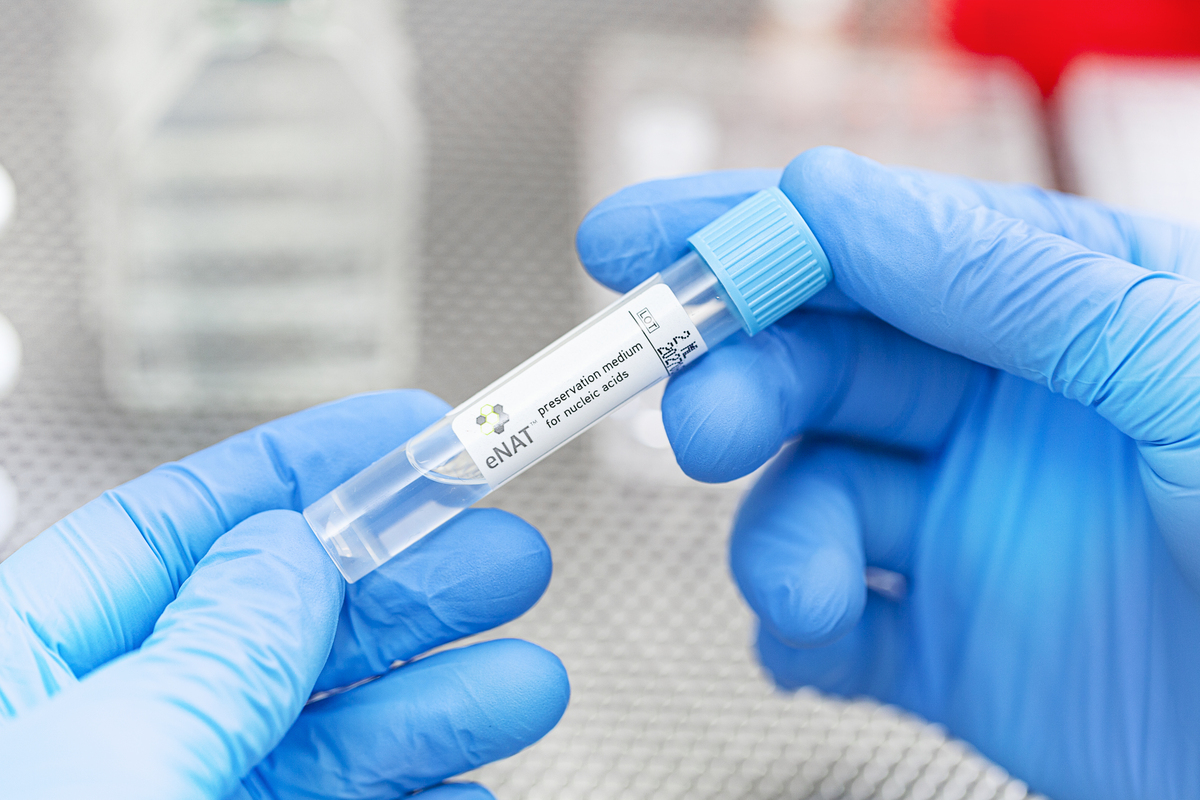
Can eNat® be processed using COPAN UniVerse®?
Yes, eNat® can be processed on COPAN’s Fully Automated Instrument for Molecular Specimen Preparation, UniVerse®. UniVerse® system revolutionizes up-front sample processing for molecular diagnostics, automatically managing accessioning, labeling, vortexing, and aliquoting into assay tubes and 96-well plates.

Is eNat® safe to use with potentially infectious specimens?
Yes, eNat® is designed to securely inactivate specimens that may contain highly infectious agents. The eNat® medium, which contains a detergent and a protein denaturant, completely neutralizes microbial viability within minutes.
A recent study published in “Viruses,” revealed that not only did COPAN’s eNat® inactivate high levels of SARS-CoV-2, but it did so particularly fast — within two minutes of contact with the medium. COPAN’s eNat® was recently fast-tracked for clearance by the FDA for Flu A RNA stabilization. The sample collection and transport system also has implied use for samples suspected of containing other viruses, including SARS-CoV-2.

Is eNat® suitable for rapid antigen testing or cell culture?
No, eNat® is not suitable for culture or rapid antigen testing.

Can eNat® be used for COVID-19 testing?
eNat® has been FDA cleared Flu A RNA stabilization. There is an implied use for samples suspected of containing SARS-CoV-2.

Where can I purchase eNat®?
Copan’s eNat® can be purchased through Copan’s online store or through our distribution partners. Click the button below to visit our online storefront.


