COPAN Announces FecalSwab® Stool Collection and Transport Device Certified Free of Nucleic Acids
March 28, 2019
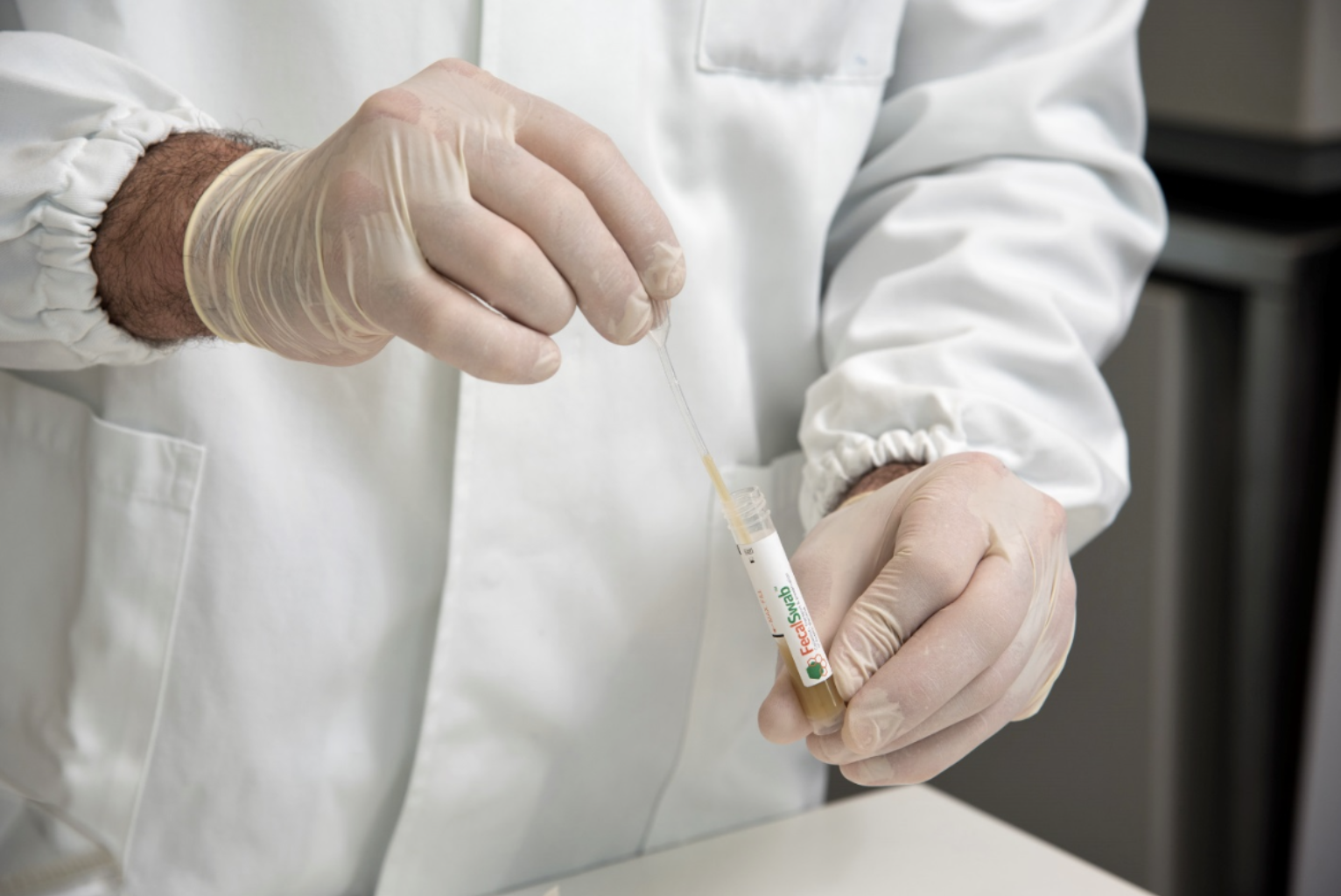 COPAN is proud to announce that FecalSwab® is the first Cary-Blair collection and preservation system supplied with a certification affirming that the medium has no detectable level of nucleic acids from the most prevalent gastrointestinal pathogens.
COPAN is proud to announce that FecalSwab® is the first Cary-Blair collection and preservation system supplied with a certification affirming that the medium has no detectable level of nucleic acids from the most prevalent gastrointestinal pathogens.
Infectious disease diagnostics is continually evolving. In particular, the application of molecular assays has steadily increased in recent years. As the field of molecular diagnostics advances, the accuracy and sensitivity of commonly used assays has increased dramatically.
Traditional Cary-Blair systems designed and intended for culture of enteric pathogens are now being widely used for new molecular diagnostics assays. This changing trend toward molecular diagnostics has revealed the problem of extraneous nucleic acid contaminants in traditional Cary-Blair transports that can potentially cause erroneous false-positive results for Vibrio and Yersinia species. COPAN FecalSwab® is the first of a new generation of Cary-Blair transport systems, developed with a broad utility in mind, meaning traditional culture methods and contemporary molecular diagnostic assays.
COPAN’s commitment to high quality is reflected on the meticulous testing on the raw materials used in the production of FecalSwab® to verify the absence of nucleic acids from relevant microorganisms. The investigation was performed using BioFire® FilmArray® GI Panel.
The BioFire® FilmArray® GI Panel is a multiplexed nucleic acid test for the simultaneous qualitative detection and identification of nucleic acids from multiple bacteria, viruses, and parasites directly from samples in Cary-Blair transport medium. The BioFire® FilmArray® GI Panel is well known for its broad spectrum and accuracy and the COPAN and BioFire® teams worked closely together to perform the QC testing.
The results confirmed the absence of detectable levels of nucleic acids of any of the organisms in the BioFire® FilmArray® GI Panel, and the BioFire™ team reviewed and confirmed these results. Each box of COPAN’s FecalSwab® is supplied with a certification affirming that the medium is free of detectable level of nucleic acids from the indicated pathogens.
“In the continuously changing field infectious disease diagnostics, users can rest assured that FecalSwab® and all COPAN products are held to the highest standards of quality. Clinicians and laboratory processionals can be confident that COPAN’s collection and transport devices effectively preserve samples to ensure the most accurate test results. After all, the quality of a clinical finding is only as sound as the quality of the patient sample” stated Norman Sharples, CEO, COPAN Diagnostics.
About COPAN Group
With a reputation for innovation, COPAN is the leading manufacturer of collection and transport systems in the world. COPAN’s collaborative approach to pre-analytics has resulted in Flocked Swabs, ESwab™, UTM® Universal Transport Medium, and laboratory automation, WASP® and WASPLab. COPAN carries a range of microbial sampling products, inoculation loops, and pipettes. For more information, visit www.copanusa.com.

