CDC Recommends Copan-Manufactured Viral Transport Media and Flocked Swabs for Collection of H1N1 (Swine Flu) Virus
May 5, 2009
As of May 5th, 2009, the World Health Organization, has officially reported 1124 cases of Influenza A (H1N1) infection in 21 different countries[i]. As the situation with the novel H1N1 (Swine Flu) virus continues to evolve, the Centers for Disease Control and Prevention (CDC) continue to update their document Interim Guidance on Specimen Collection and Processing for Patients with Suspected Influenza A (H1N1) Virus. The objective of this document is to provide “guidance on appropriate specimen collection, storage, processing, and testing for patients with suspected swine-origin influenza A (H1N1) virus infection[ii].”
One of the most
widely used methods for detection of H1N1 flu virus is to collect a nasopharyngeal
swab sample. The CDC recommends the use
of synthetic tip swabs, such as Flocked Swabs, which exhibit a superior
recovery of viruses. Several independent scientific studies published in the Journal
of Clinical Microbiology and presented at Clinical Virology Symposium show that
the quality of the sample taken using Copan Flocked Swabs is comparable to the
quality of a sample collected from a nasopharyngeal aspirate or nasal was, which
are considered the gold standard for upper respiratory virus samples. The open
structure of Copan Flocked Swabs acts like a soft brush that efficiently
dislodges and collects cells and mucus, then releases the sample immediately
when put in a viral transport medium. In
Ontario, the Agency for Health Protection and Promotion also lists Flocked
Swabs as the preferred type of swab for respiratory virus sample collection on
its latest version of LABstract on “H1N1 Flu Virus (human swine influenza), Updated
Guidance for Laboratory Testing, Hospital Setting[iii].”
Copan Flocked Swabs are easy to use, are less invasive and cause minimal
discomfort for the patient. Copan holds a worldwide patent on Flocked Swabs.
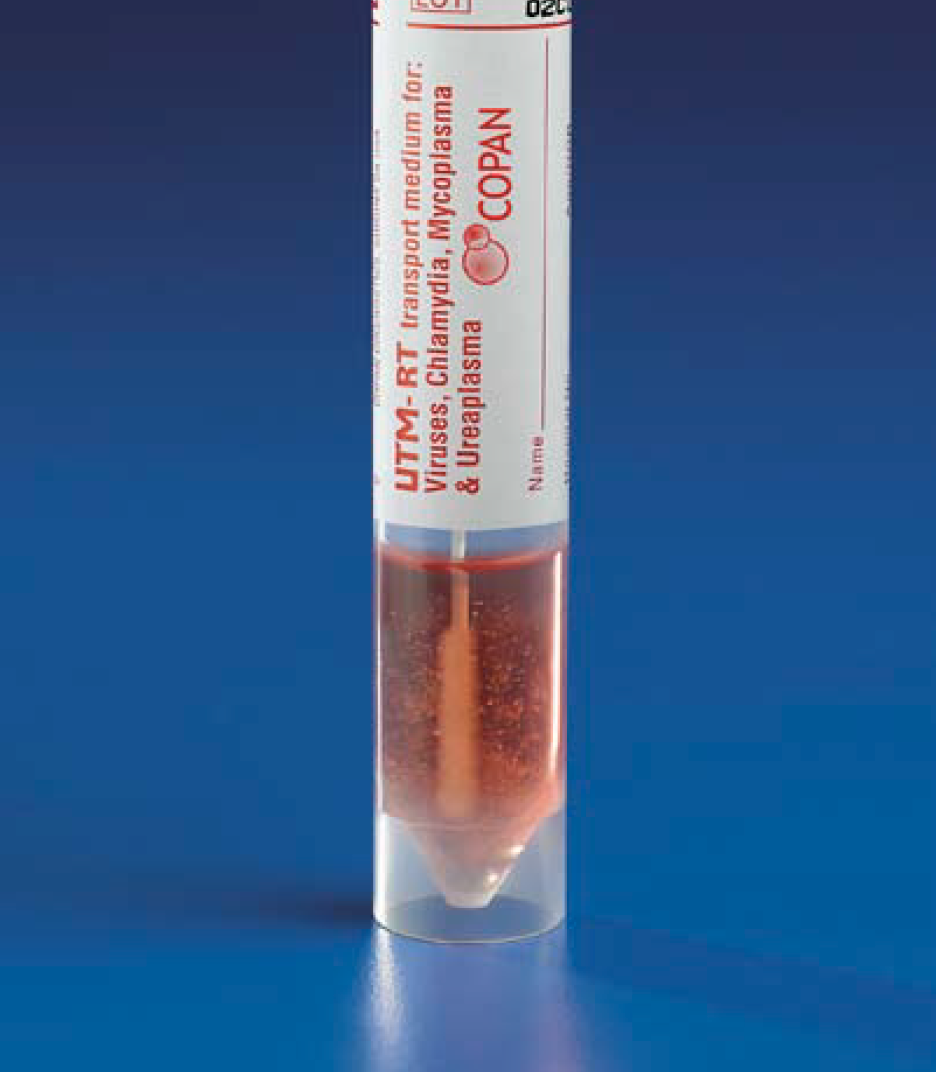
To transport H1N1 viral specimens
collected using swabs, the CDC also recommends that the “swab specimen
collection vials should contain 1 – 3ml of viral transport medium (e.g.
containing, protein stabilizer, antibiotics to discourage bacterial and fungal
growth, and buffer solution[iv]),”
such as the BD Universal Viral Transport System, which is manufactured by Copan
and distributed in different parts of the world under the Copan brand name
Universal Transport Medium (UTM). Copan UTM is room temperature stable and
available in vials containing 1 or 3 ml of transport medium for different applications.
For more information about Copan UTM
visit: http://www.copanusa.com/products/utm/
[i] Influenza A(H1N1) –
update 14. 4 May 2009. http://www.who.int/csr/don/2009_05_04a/en/index.html
[ii] Interim Guidance on
Specimen Collection, Processing, and Testing for Patients with Suspected
Swine-Origin Influenza A (H1N1) Virus Infection. April 30, 2009. http://www.cdc.gov/h1n1flu/specimencollection.htm
[iii] LAB-SD-059-001 –
Influenza A (H1N1) – Updated Guidance for Laboratory Testing Hospital Setting-
final PHL 03- 05-09
[iv] Interim Guidance on
Specimen Collection, Processing, and Testing for Patients with Suspected
Swine-Origin Influenza A (H1N1) Virus Infection. April 30, 2009. http://www.cdc.gov/h1n1flu/specimencollection.htm
About Copan Diagnostics,
Inc.
With a reputation for innovation in preanalytics, Copan Diagnostics is the leading manufacturer of collection and transport systems in the world. Copan offers a complete range of microbial sampling products used for traditional culture analysis and for molecular diagnostic assays, as well as producing innovations, like the patented Flocked Swabs, Universal Transport Medium and ESwab. The company manufactures a comprehensive line of laboratory consumables comprising of inoculation loops, needles, spreaders and transfer pipettes. Copan also produces WASP: Walk-Away Specimen Processor, a new revolutionary system for automatic planting and streaking of microbiology samples. For more information about Copan, visit www.copanusa.com

