COPAN Flocked Swabs and UTM™ Viral Transport Media Available in Response to pH1N1 Upsurge
January 10, 2014
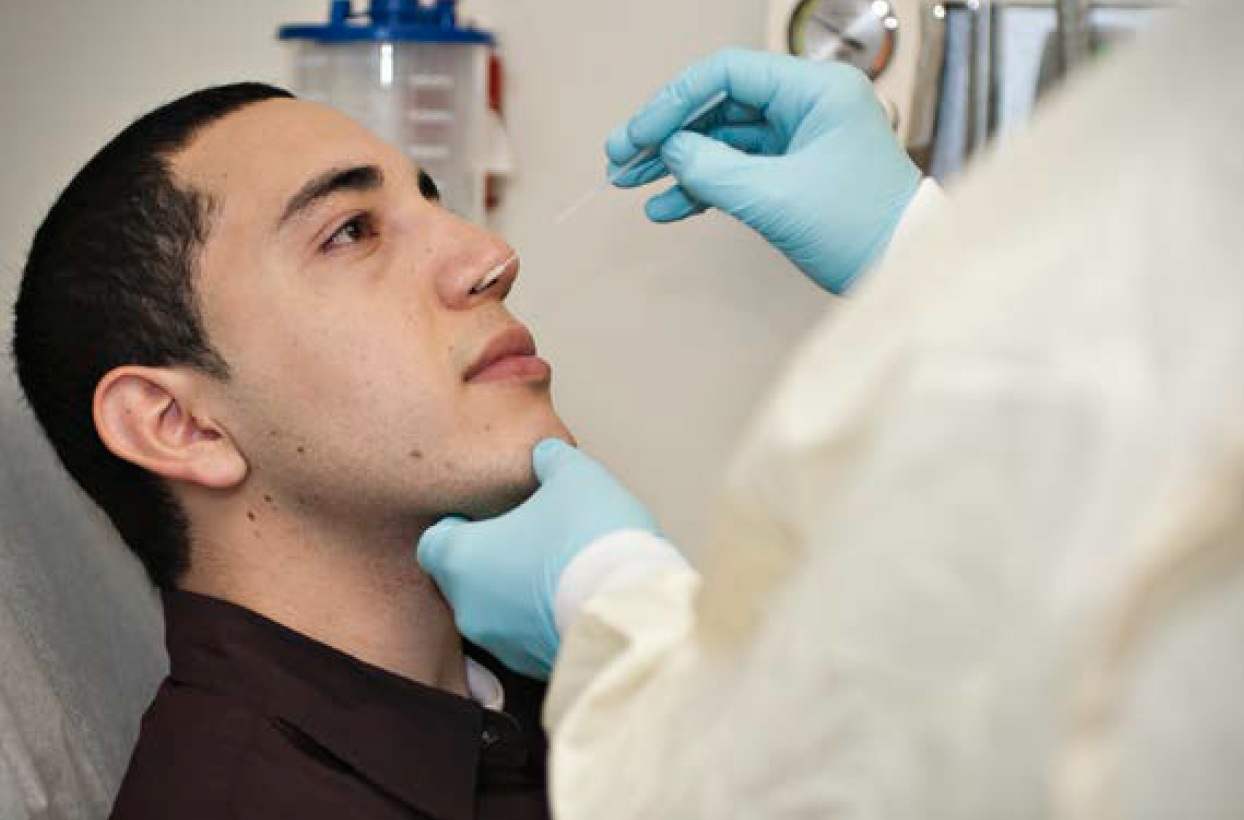
Influenza season is well underway with nearly half the country reporting flu cases. Close to half of the cases being reported in the US are associated with the strain pH1N1 according to the Center for Disease Control (CDC). The CDC issued an alert about early reports of pH1N1- associated illnesses for 2013-14 Influenza Season with severe cases of respiratory illnesses affecting young and middle-aged adults. Originally called swine flu, H1N1 emerged during the 2009-2010 flu season, and it has maintained a human presence in each flu season since then. In Europe, influenza detection has also risen during the first week of 2014, with the true picture still unclear due to underreporting of detections from some countries during the holidays.
COPAN’s flu sample collection kits comprise of UTM™ viral transport media and a patented flocked swab, which has been shown in many independent clinical studies to improve the results of respiratory virus diagnostic tests by collecting more patient specimen and delivering the entire sample into the assay platform or cell culture tube. UTM™ and a COPAN’s flocked swab is the optimum kit combination for virology culture, rapid antigen testing, and DFA testing for influenza virus; and the use of the combined products is also a universal open platform viral collection and transport system that has been successfully used for EIA, PCR and other molecular based assays.
“During the 2009 H1N1 outbreak, COPAN invested tremendously in production capacity to meet the global demand, so we are more than prepared to handle the increased demand we are seeing now” says Norman Sharples, CEO of COPAN Diagnostics, Inc. “As the leader in collection and transport systems, we have the infrastructure needed to meet the demand in a timely manner. Our production of flocked swabs and UTM™ includes shifts 24 hours per day, and we are working closely with our distribution partners to ensure they continue to have plenty of stock,” Sharples confirms.
In cases of influenza, collecting a proper sample in a timely manner is crucial to ensure the patient is treated within the therapeutic window. COPAN flocked swabs, as opposed to traditional nasopharyngeal washes or aspirates, facilitate easier sample collection and produce comparable results. By using a FLOQSwab and UTM™, the sample collection can be done in outpatient departments and remote sites and can be safely transported to the laboratories for testing. To optimize results, COPAN has worked with scientists and physicians, as well as the Joint Commission, to develop several training videos on the correct procedures to properly collect samples for the diagnosis of respiratory virus infections, like pH1N1. These videos are available on the company’s website and are free of charge.
About COPAN Diagnostics, Inc.
With a reputation for innovation, COPAN is the leading manufacturer of collection and transport systems in the world. COPAN’s collaborative approach to pre-analytics has resulted in Flock Swabs, ESwab, UTM™ and modular laboratory automation, WASP® and WASPLab. For more information about COPAN Diagnostics, visit http://www.copanusa.com.

