Research RoundUp: FecalSwab® and Molecular Testing
March 7, 2023

Welcome to Research Roundup, where COPAN will feature a comprehensive review and commentary on recent scientific publications covering a particular topic of interest. COPAN’s Research Roundup is authored by Dr. Susan Sharp, COPAN’s Scientific Director!
In this edition of Research Roundup, Dr. Sharp summarizes research that explores FecalSwab®, a multiple purpose fecal transport system for manual and automated culture setup and for enteric molecular diagnostics.
About FecalSwab®
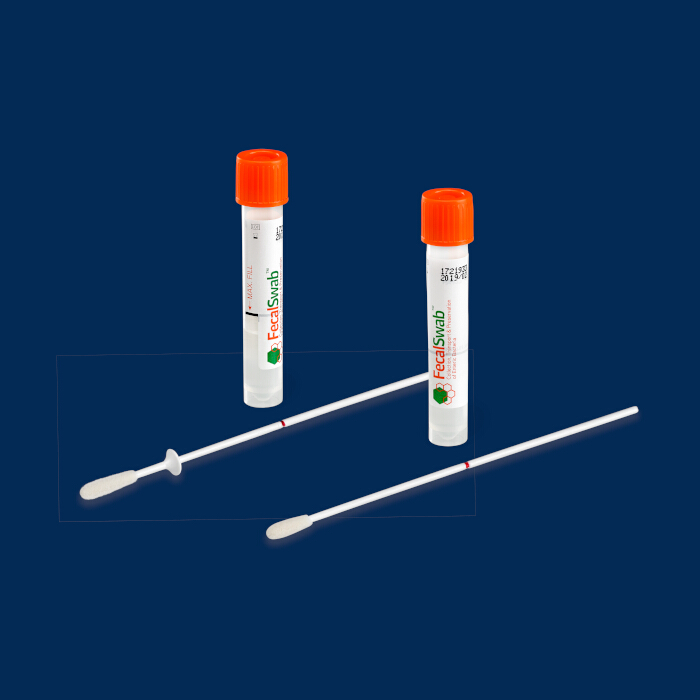
COPAN FecalSwab® consists of a tube containing 2mL of Cary-Blair medium and a flocked swab. The system is FDA-cleared and designed for preserving and transporting fecal specimens and rectal swabs to culture enteric bacterial pathogens. The Cary-Blair medium is compatible with a variety of enteric molecular testing assay platforms, and numerous laboratories throughout the country have added the Copan FecalSwab® to their armamentarium of collection devices.
In addition to the standard configuration, the kit may alternatively include a flocked collection swab with plastic ring marker on the shaft which helps clinicians to visualize the maximum depth for specimen collection by rectal sampling. The FecalSwab® has a unique, compact size compared to conventional stool specimen transport containers, which makes it highly convenient for storage and transportation. Additionally, it is compatible with automated culture and molecular testing platforms.

In late 2022, COPAN obtained FDA clearance for the use of FecalSwab® with both the BD MAX™ Enteric Bacterial Panel and the BD MAX™ Extended Enteric Bacterial Panel, making it the only collection and transport device authorized by the FDA for molecular detection of enteropathogens (bacteria, viruses, and parasites) using BD MAX™ and Extended Enteric Panels, as well as preserving microbial vitality for various applications.
Prior to FDA clearance of FecalSwab® with the BD MAX™ system two independent studies indicated the successful use of the sample collection system with BD’s enteric platform.
The Research Roundup
Evaluation of the FecalSwab® for Stool Specimen Storage and Molecular Detection of Enteropathogens on the BD MAX™ System
In 2020, a study was conducted to assess the effectiveness of FecalSwab® in testing 186 unpreserved stool specimens in the BD MAX™ system, using both viral and bacterial enteric panels. Out of the 186 patients, 30 (16.1%) had no detected pathogen, while 156 (83.9%) tested positive for either a virus (133) or bacteria (93).
The study found an overall agreement of 99.3% and 99.5% for the viral and bacterial panels, respectively, between FecalSwab® and fresh stool specimens, indicating that the two methods have comparable detection rates. Among the 7 samples that showed discordant results, FecalSwab® specimens were positive for a pathogen target while fresh stool was negative.
Additionally, the study examined the stability of stool specimens stored in FecalSwab® for up to 14 days at 4°C, 22°C, and 35°C. While most viral targets remained detectable even after 2 weeks of storage at 4°C, the detection of enteric bacteria was inconsistent when stored at 22°C or 35°C.
The authors concluded that FecalSwab® performs equally well as fresh stool, and it maintains molecular detection sensitivity when stored at 4°C. Therefore, FecalSwab® is a viable option as a collection and storage device for enteropathogen diagnostics on the BD MAX™ system. (Richard-Greenblatt)

BD MAX™ Evaluation of Copan FecalSwab® Preserved Stool Specimens with the BD MAX™ Enteric Bacterial Panel and the BD MAX™ Extended Enteric Bacterial Panel
A second study was conducted to evaluate the effectiveness of FecalSwab® in preserving stool specimens for testing with the BD MAX™ Enteric Bacterial Panel and the BD MAX™ Extended Enteric Bacterial Panel.
The study aimed to achieve two objectives: first, to determine the ideal volume of FecalSwab®-preserved stools in comparison to the recommended Meridian Para-Pak Cary-Blair medium for these assays, and second, to compare the performance of FecalSwab® and PP in the assays.
For the first objective, ATCC strains of enteric organisms were inoculated with stool volumes of 10, 25, and 50 μL and tested. The results showed that there was no difference between 25-μL and 50-μL volumes from the FecalSwab® when compared with the 10-μL, and as the 50-μL volume perform equally or better than the 10-μL, a 50 μL volume of FecalSwab® was used for the testing of clinical specimens.
For the second objective, 144 unpreserved stool specimens were transferred to Para-Pak and FecalSwab® and tested using 10 μL of Para-Pak and 50 μL of FecalSwab® media.

The results showed a 100% agreement between Para-Pak and FecalSwab® in the two molecular assays. This study established that FecalSwab® is a good and reliable alternative to the bulky Cary-Blair transport device for preserving enteric pathogenic bacterial nucleic acid in fecal specimens from patients suspected of gastrointestinal infection with the BD MAX™ Enteric Bacterial Panel on the BD MAX™ System. (Rojas)
The COPAN FecalSwab® collection, transport and preservation system is designed for collecting and transporting rectal swab and fecal specimens while maintaining the viability of enteric pathogenic organisms during transport from the collection site to the laboratory for culture. FecalSwab® and generic Cary-Blair transports are named as suitable sample collection devices in some IFU pack inserts of enteric molecular assay platforms.
While not currently FDA cleared, another independent study has concluded that FecalSwab® can be utilized with stool specimens tested in the BioFire® FilmArray® GI Panel. The study also found that FecalSwab® optimizes the collection and transport of gastrointestinal pathogens, allowing for rapid diagnosis of gastrointestinal diseases.

Evaluation of the New FecalSwab® System for Maintaining Stability of Stool Samples Submitted For Molecular Tests
In 2017, a study was published that evaluated the effectiveness of FecalSwab® in detecting 22 pathogens using the Biofire® FilmArray® GI molecular panel, which was tested on 103 stool specimens.
The study tested stools using two different protocols:
- SC: The standard of care which used fresh stool samples in Cary-Blair medium and;
- FS: FecalSwab® which involved transferring fresh stool samples to Cary-Blair media using the provided flocked swab.
The study found that out of the 103 stool specimens tested, 33 (32%) showed no detected pathogen, while 70 (68%) tested positive for 81 and 82 pathogens in the SC and FecalSwab® protocols, respectively, with an overall agreement of 97.1% (100/103 specimens). Among the three discrepant specimens, two showed positive results for C. difficile only in the FecalSwab® protocol, while one tested positive for Cryptosporidium only in the SC protocol.
In addition, a stability analysis was conducted to determine the efficacy of both protocols in preserving target nucleic acid for 24 hours at room temperature. This analysis showed that 20 out of 25 specimens with the standard of care (SC) protocol and 23 out of 25 specimens with FecalSwab® (FS) protocol had concordant results as compared to the 0-hour results.
Based on these findings, these authors concluded that the FecalSwab® can be used with stool specimens tested in the FilmArray® GI Panel, and that FecalSwab® optimizes the collection and transport of GI pathogens, enabling rapid diagnosis of gastrointestinal diseases. (Silbert)
References & Citations
1.) Silbert S, Gostnell A, Kubasek C, Widen R. 2017. Evaluation of the new FecalSwab system for maintaining stability of stool samples submitted for molecular tests. J Clin Microbiol 55:1588 –1590. https://doi.org/10 .1128/JCM.00273-17.
2.) Richard-Greenblatt M, Rutherford C, Luinstra K, Cárdenas AM, Pang XL, Jayaratne P, Smieja M. 2020. Evaluation of the FecalSwab for stool specimen storage and molecular detection of enteropathogens on the BD Max system. J Clin Microbiol 58:e00178-20. https:// doi.org/10.1128/JCM.00178-20.
3.) Rojas HF, Lima A, Kubasek C, Gostnell A, Silbert S. Evaluation of Copan FecalSwab™ preserved stool specimens with the BD MAX™ Enteric Bacterial Panel and the BD MAX™ Extended Enteric Bacterial Panel. DMID 97 (2020) 115055.
FecalSwab® Patented Sample Collection and Preservation System for Enteric Bacteria
Stay Informed
With tens of thousands of scientific papers published each year, it is hard to stay informed on the relevant research. Each month COPAN’s Research Roundup will feature a comprehensive review and commentary on recent scientific publications covering a particular topic by experts in the field to help keep you up to date on the latest research. Sign up today so you don’t miss the latest findings!
About Dr. Susan Sharp

Dr. Sharp has been a clinical microbiologist for over 30 years and joined Copan Diagnostics as the Scientific Director in 2018.
Dr. Sharp is actively involved with the American Society for Microbiology (ASM) and is a past-president of ASM. She is a Diplomat of the American Board of Medical Microbiology and a Fellow in the American Academy of Microbiology.
Dr. Sharp currently serves as a member of the Board of the Clinical and Laboratory Standards Institute, a member of the Sub-Committee for Antimicrobial Susceptibility Testing for CLSI, a member of the CLSI Microbiology Expert panel, is a Governor for the American Academy of Microbiology, and is a past member of the Board of Scientific Councilors for the Office of Infectious Diseases at the CDC.
She has lectured both nationally and internationally and has published numerous scientific articles and book chapters in the field of clinical microbiology. Her most prominent area of interest has centered on cost-effective, clinically-relevant diagnostic microbiology.
About COPAN Diagnostics, Inc.
With a reputation for innovation, COPAN Diagnostics is part of COPAN Group, the leading manufacturer of collection and transport systems in the world. COPAN’s collaborative approach to pre-analytics has resulted in Flocked Swabs, ESwab®, UTM®: Universal Transport Medium™, and laboratory automation, WASP® and WASPLab®. COPAN carries a range of microbial sampling products, inoculation loops, and pipettes. For more information, visit www.copanusa.com
Medical Disclaimer: This page is for informational purposes only and should not be a substitute for professional medical advice, diagnosis, or treatment. Always refer to the Centers for Disease Control & Prevention, professional healthcare organizations, or other qualified healthcare providers for current guidance.




