ESwab® FAQ
This FAQ page provides information on ESwab®, a liquid-based multipurpose collection and transport system for microbiology specimens, covering topics such as what ESwab® is, how to use it, what it supports, stability requirements, applications, and availability.
Contents
- What is ESwab®?
- What is a Flocked Swab?
- What does the “E” in ESwab® stand For?
- What Types of Bacteria Does ESwab® Support?
- How Long Does ESwab® Support Bacteria?
- What are the Stability and Transport Requirements of ESwab®?
- I’ve heard that ESwab® is a Multipurpose Transport System. What Does this Mean?
- What is the Shelf Life of ESwab®?
- Can ESwab® be Used for Multiple Tests?
- Can ESwab® be Used for Manual Plating?
- Can ESwab® be Used for Gram Stains?
- Does ESwab® Work with Automation?
- Can ESwab® be Used for Molecular Applications and Rapid Antigen Tests?
- How is the Sample Collected Using ESwab®?
- What is the Benefit of the ESwab® Capture Cap?
- Must the Swab Be Used to Perform My Tests?
- How is ESwab® Sterilized?
- What are the Most Common Types of ESwab®?
- Where Can I Order ESwab®?
- Is ESwab® Suitable for COVID-19 Testing?
- Do You Have Other Questions about ESwab® FAQ?
What is ESwab®?
- ESwab® is a liquid-based multipurpose collection and transport system for Microbiology. ESwab® combines a COPAN-invented flocked swab with 1mL of Liquid Amies in a plastic, screw cap tube. The innovative system elutes over 90% of patient specimen into the liquid medium. The result is evidence-based, improved pathogen recovery, expanded testing capabilities, and better patient care.
- The FDA cleared collection and transport system is suitable for aerobic, anaerobic and fastidious bacteria maintaining viability for up to 48 hours at room or refrigerator temperature (Neisseria gonorrhoeae survival at 24 hours per CLSI standard).
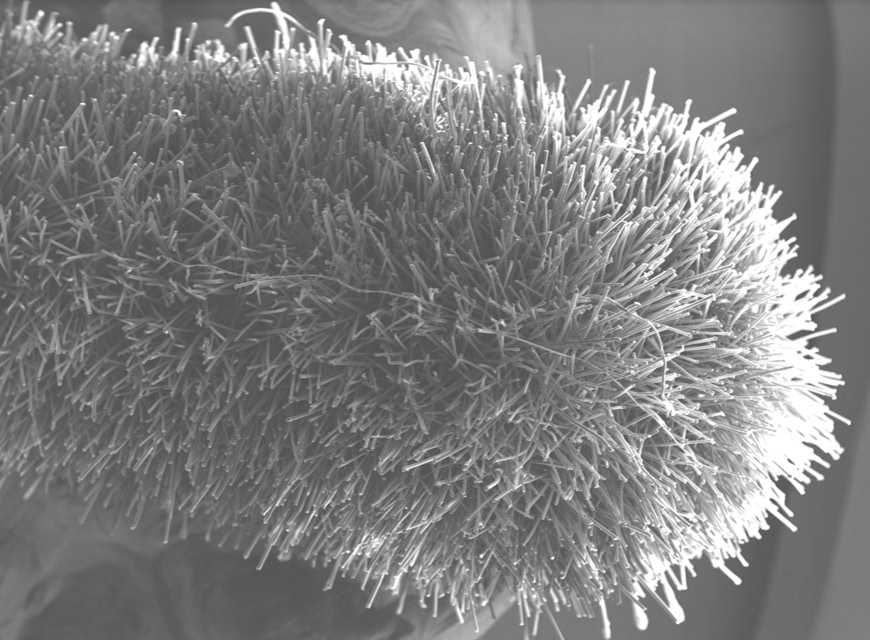
What is a Flocked Swab?
Invented by COPAN, flocked swabs are a special type of swab produced with an applicator tip that is spray-coated with short Nylon® fibers arranged perpendicularly forming a soft, velvet brush that allows for improved collection of cell samples.
Capillary action between the Nylon® fiber strands facilitates strong hydraulic uptake of liquid samples. Unlike traditional swabs made with materials that can trap the specimen, flocked swabs use a thin absorbent layer of Nylon® fibers with no internal core, so the sample stays close to the surface, allowing fast and complete elution.
What does the “E” in ESwab® stand For?
Elute! The flocked swab allows for fast and complete elution of samples.
What Types of Bacteria Does ESwab® Support?
ESwab® is FDA cleared for collection, preservation, and transport of specimens to maintain the viability of aerobic, anaerobic, and fastidious bacteria at refrigerator and room temperature.
How Long Does ESwab® Support Bacteria?
ESwab® has been tested and validated in full compliance with CLSI M40-A2: Quality Control of Microbiological Transport System Standard. The FDA cleared collection and transport system is suitable for aerobic, anaerobic and fastidious bacteria maintaining viability for up to 48 hours at room or refrigerator temperature (Neisseria gonorrhoeae survival at 24 hours per CLSI standard).

What are the Stability and Transport Requirements of ESwab®?
Samples should be transported to the laboratory as soon as possible. If immediate delivery or processing is delayed, then specimens should be refrigerated at 4 – 8°C or stored at room temperature (20 – 25°C) and processed within 48 hours, except for Neisseria gonorrhoeae cultures, which should be processed within 24 hours per the CLSI standard.
I’ve heard that ESwab® is a Multipurpose Transport System. What Does this Mean?
ESwab® is a versatile collection and transport system, which can be used for traditional culture for aerobes, anaerobes, and fastidious bacteria. Independent studies have shown ESwab® is successfully used for the recovery of mycobacteria, fungi, and trichomonas. Studies are available upon request. Additionally, ESwab® can be used for Gram stains, automation, rapid antigen testing*, and molecular assays*.
*Always read the manufacturer’s package insert for specific instructions regarding specimen collection and transport for the type of test kit being used.
What is the Shelf Life of ESwab®?
ESwab® is shelf stable for 15 Months.

Can ESwab® be Used for Multiple Tests?
Yes! Because ESwab® provides 1mL of homogenous liquid sample, it provides up to 10 aliquots of identical sample to perform multiple tests.
Can ESwab® be Used for Manual Plating?
Yes! Manual plating can be performed either by using the swab to inoculate or by using a sterile pipette and inoculating sample onto the media.
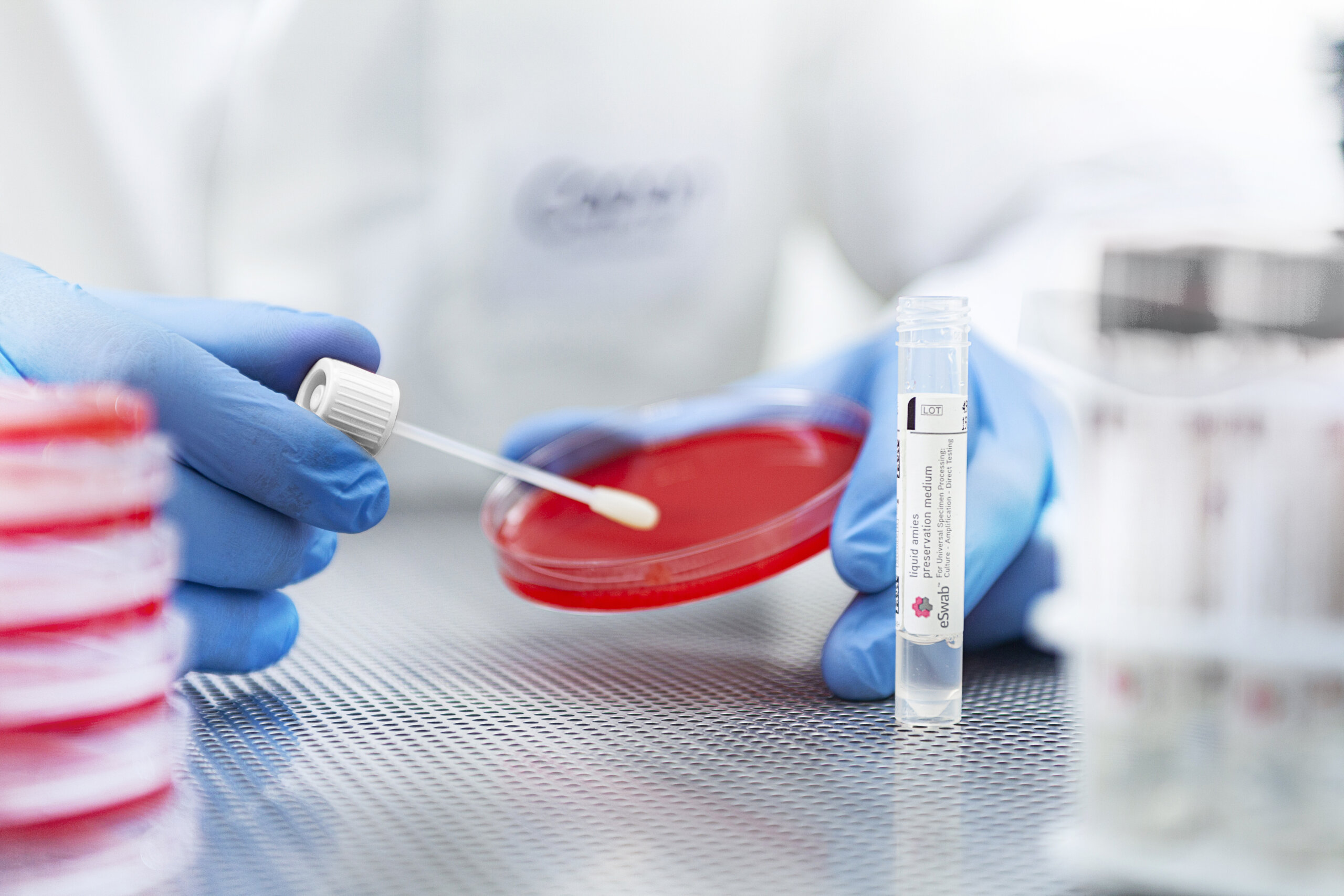
Can ESwab® be Used for Gram Stains?
Yes! ESwab® creates more consistent Gram stains since it provides a more homogenous suspension than standard swabs. ESwab® should be vortexed for 5 seconds, then using a sterile pipet, transfer 30 µL (1-2 drops) of the sample. Methanol fixation is recommended.
Does ESwab® Work with Automation?
Yes! ESwab® is approved for use with WASP®. For compatibility of ESwab® with other automated platforms please refer to the documentation from the respective manufacturer.

Can ESwab® be Used for Molecular Applications and Rapid Antigen Tests?
Always consult manuals, product inserts, and instructions for use for the appropriate use of all products. ESwab® has been increasingly qualified by leading assay manufacturers for use with their molecular and rapid antigen tests. ESwab® has been used successfully for rapid antigen testing and for molecular-based assays with self-verification. To learn more about ESwab® versatile use, visit the scientific studies section of this website.

How is the Sample Collected Using ESwab®?
At the point of care, the ESwab® cap is unscrewed and removed. After the sample is collected from the patient, the swab is inserted to the bottom of the ESwab®tube and the swab shaft is broken at the marked, molded breakpoint. The cap is screwed back onto the tube. The sample is labeled and sent to the lab.
ESwab® Sample Collection Guides
Nasopharyngeal Collection Guide
Oropharyngeal Collection Guide
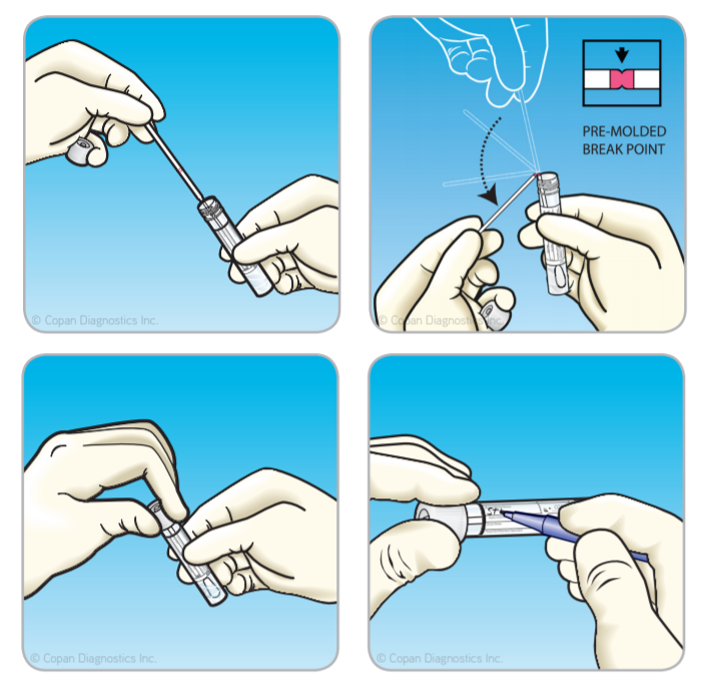
What is the Benefit of the ESwab® Capture Cap?
- At the point of care, the ESwab® cap is unscrewed and removed. After the sample is collected from the patient, the swab is inserted to the bottom of the ESwab® tube and the swab shaft is broken at the marked, molded breakpoint. The cap is screwed back onto the tube. The sample is labeled and sent to the lab.
- The capture cap feature allows the operator to conveniently remove the swab and perform various microbiology analyses using the cap as a handle to hold and manipulate the swab.

Must the Swab Be Used to Perform My Tests?
No. Because the sample is eluted into the media, the specimen is in the liquid, not on the swab. While the swab may be used as a transfer device, many laboratories prefer a loop or a transfer pipet to take an aliquot for testing.

How is ESwab® Sterilized?
- ESwab® is sterilized using gamma irradiation. Gamma irradiation is a form of electromagnetic radiation—like microwaves and x-rays, but with higher energy. It is a commonly used method for sterilizing medical devices. During the sterilization process, gamma rays pass through the product and kill any microorganisms by destroying bacterial and viral DNA. The treatment does not leave behind any form of residue in the product. As well as medical products, gamma irradiation is also used to sterilize food, dental, and household products. The FDA, CDC, WHO, and USDA have all evaluated and endorsed irradiated food and other products’ safety for many years.
- In addition to state and federal Nuclear Regulatory Commission (NRC) supervision, the manufacture and gamma sterilization of medical devices, like ESwab®, are tightly regulated by the FDA. Therefore, gamma irradiation processing is performed with appropriate validation and quality assurance controls and documents to assure effective and safe processing.
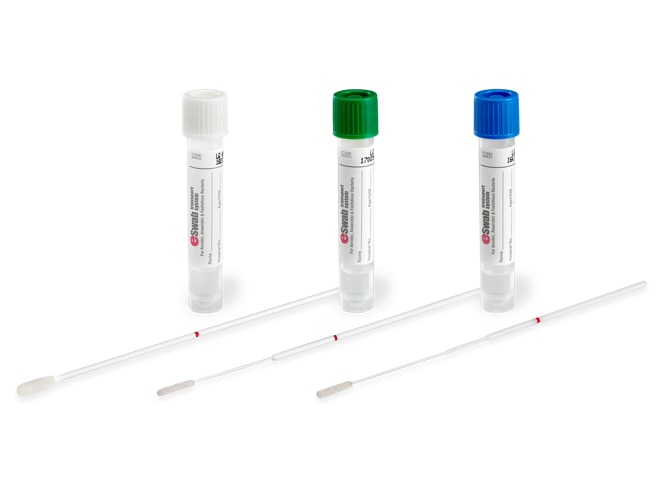
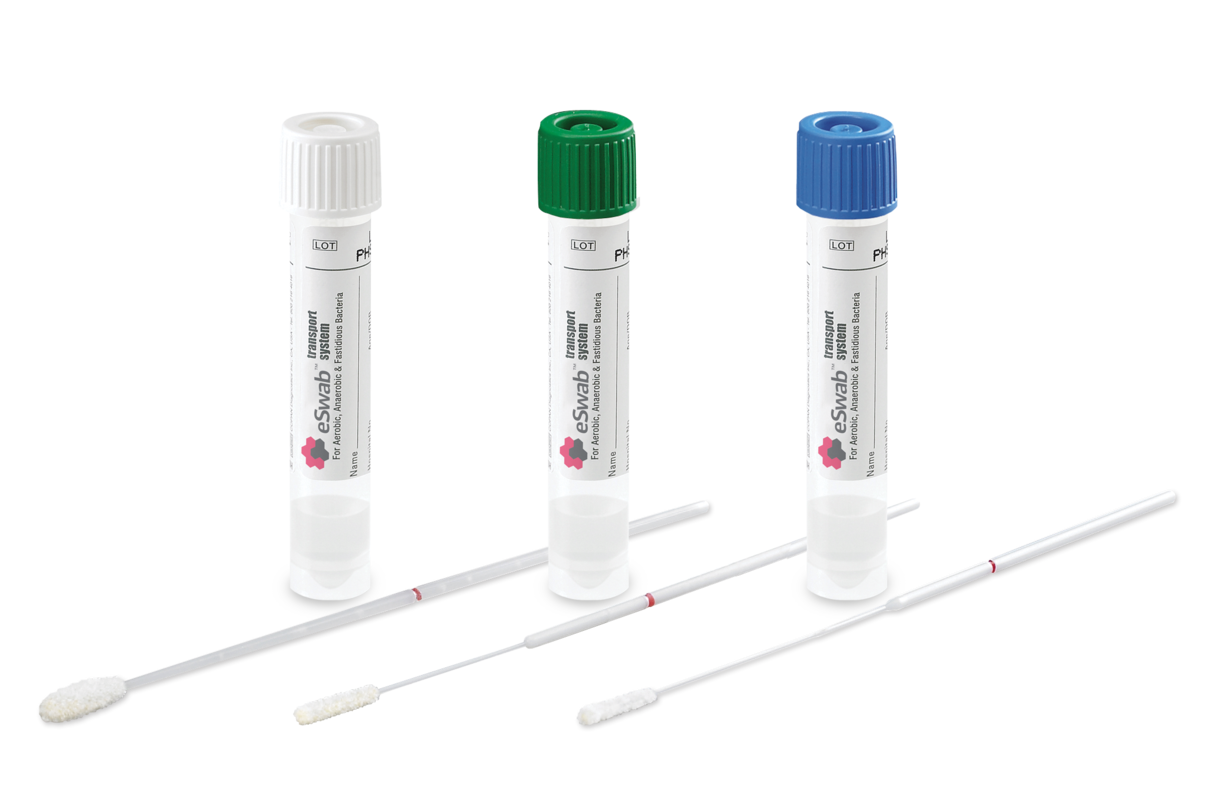
What are the Most Common Types of ESwab®?
Different size swabs may be appropriate for different collection sites. For example, minitip swabs may be more appropriate for smaller collection sites or pediatric sample collections.
There Are 3 Swab Types:
Where Can I Order ESwab®?
In the United States, Copan does not sell sample collection products directly to clinical laboratories. Our products are available through our distribution partners.

Is ESwab® Suitable for COVID-19 Testing?
In the absence of viral transport medium or UTM®, liquid Amies media, a medium found in COPAN ESwab®, may be used. COPAN has compiled a COVID-19 resource page to facilitate answers to some of the frequently asked questions being received. Here you’ll find easy access to the evolving regulatory guidelines published by the CDC and FDA, COPAN’s distributor part numbers, and other resources about critical products that can be used for collecting, handling, and transporting specimens suspected of COVID-19.
*Always read the manufacturer’s package insert for specific instructions regarding specimen collection and transport for the type of test kit being used.
** Check for current guidance with the FDA, CDC, or other local, state, or federal governing bodies for the latest recommendations.


